Respirology
American Thoracic Society (ATS) 2013 International Conference
Particle-Engineering Delivery Systems Showing Versatility for Antibiotic Delivery in Lung Diseases
Philadelphia – Particle engineering technology is coming of age for topical delivery of antibiotics to patients with chronic lung diseases, according to separate clinical trials presented at this year’s American Thoracic Society (ATS) International Conference. In one study, the goal was to evaluate the efficacy and safety of delivering tobramycin in dry powder form to the lungs of children with cystic fibrosis (CF). In the other, lung deposition of dry powder inhaled (DPI) ciprofloxacin was measured in adults with chronic obstructive pulmonary disease (COPD). Both studies suggested that this technology is highly effective and safe for delivering therapeutic levels of topical antibiotics to lung tissue.
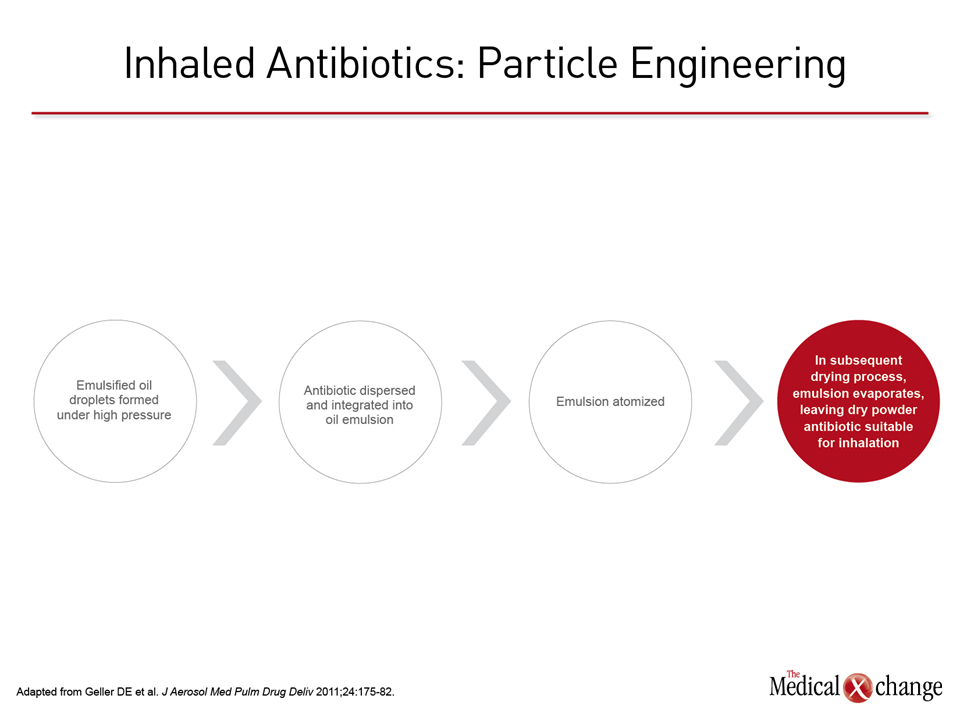
Two studies on the delivery of antibiotics to patients with chronic lung diseases were conducted with the same device, using PulmoSphere™ technology. The system is designed to optimize particle size and weight in the context of inhalation aerodynamics to improve dispersion of the dry powder antibiotic. The technology is based on an emulsion-based spray-drying process that produces light porous particles (Fig. 1). The studies presented here suggest that the new technology may permit delivery of topical antibiotics with similar or greater efficacy but far greater convenience than nebulizers, which it is designed to replace. The more important of the studies was the one conducted with tobramycin in cystic fibrosis (CF). In this study, which randomized 62 children with CF and documented Pseudomonas aeruginosa infection to tobramycin or placebo inhalation, there were highly significant reductions in sputum loads of P. aeruginosa (P=0.002) and improvements in lung function (P<0.001) for those in the tobramycin group. “The primary objective of the core study was to evaluate efficacy as relative change in FEV1% predicted from baseline to Day 29,” explained Dr. Ivanka Galeva, Alexandrovska Hospital, Sofia, Bulgaria. Senior author of the Establish Tobramycin Dry Powder Efficacy Trial (EDIT), Dr. Galeva reported that the significant improvement in FEV1 at day 29 persisted during the extension study that included three additional cycles of treatment (28 days on therapy, 28 days off). The extension provided an opportunity to demonstrate sustained suppression of P. aeruginosa with an acceptable safety profile. None of the serious adverse events identified in the study were considered to be drug related. In the phase 1 chronic obstructive pulmonary disease (COPD) study, “scintigraphy data showed that ciprofloxacin dry powder for inhalation (DPI) resulted in high, reproducible drug deposition in the lung,” reported a team of collaborating investigators from institutions in Germany. In this study, 6 patients with COPD, 6 patients with non-cystic fibrosis bronchiectasis (NCFB) and 12 healthy volunteers were evaluated after receiving radiolabelled ciprofloxacin DPI. Peripheral lung deposition was actually greater in patients with COPD and NCFB relative to the healthy volunteers. However, the more important result was an encouraging level of ciprofloxacin bioavailability, which was observed at levels that would be anticipated to provide a high degree of antibiotic activity. Clinical endpoint trials are planned. Particle engineering has grown substantially in sophistication. A better understanding of particle size in relation to inhalational aerodynamics has been critical to the development of the PulmoSphere™ technology as well as other systems being developed to deliver topically-active agents to lung tissue.