Cardiology
American College of Cardiology 64th Annual Scientific Session & Expo (ACC.15)
Emerging Outcomes Data with PCSK9 Inhibitors Show Cardiovascular Risk Reductions in Line with LDL Control
San Diego – The same relationship between lipid lowering and cardiovascular risk reductions established with statins is being seen with the novel PCSK9 inhibitors, according to data presented at the annual meeting of the ACC. The findings, although preliminary, provide promise for the large proportion of high-risk patients who are not reaching treatment goals on statins alone or who are intolerant to statins. So far, safety data in the large trial programs with the PCSK9 inhibitors have yet to identify any major adverse events.
Background
In Canada and elsewhere, almost half of patients at high-risk for cardiovascular (CV) events are not reaching LDL-cholesterol (LDL-C) goals, even when on high-intensity statins (Fig. 1). The emerging evidence of CV protection is intensifying attention to the ongoing outcomes trials designed to provide definitive proof of clinical benefit from PCSK9 inhibitors. There is an immediate need for an alternative to statins. The reasons that patients fail to reach treatment goals on statins range from inadequate lipid-lowering effect to intolerance.
CV Outcomes and PCSK9 Inhibitors
“We are seeing very large decreases in LDL-C with PCSK9 inhibitors being associated with very large decreases in events,” reported Dr. Marc S. Sabatine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA. The reduction in CV events has been proportional with the LDL-C reduction previously observed with statins. Based on current data, “the key point is that statin and non-statin drugs have very similar CV efficacy, and I think that is great news.”
“We are seeing very large decreases in LDL-C with PCSK9 inhibitors being associated with very large decreases in events.”
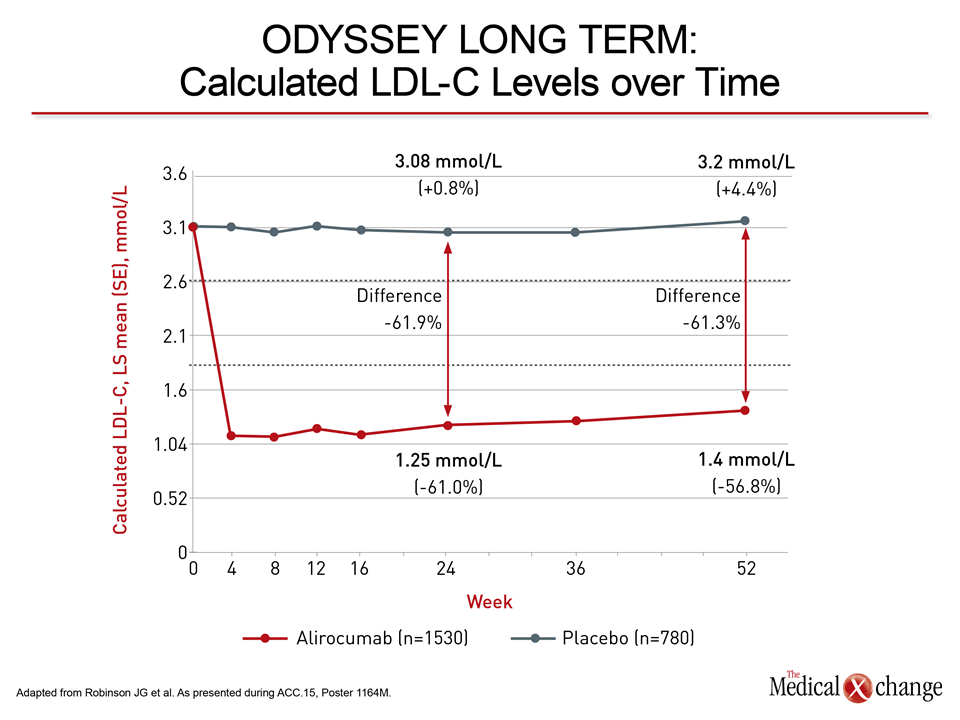
The new data were generated with alirocumab and evolocumab, the PCSK9 inhibitors that have reached late stages of clinical testing. Reductions in CV events have now been observed in long-term studies with both. Alirocumab provided the first evidence of CV protection with the phase 3 ODYSSEY LONG TERM trial. As presented at the 2014 American Heart Association (AHA) Scientific Sessions, 2341 high-risk patients were randomized to the PCSK9 inhibitor or placebo on top of current therapy. At 24 weeks, alirocumab produced a 61.9% reduction in LDL-C relative to placebo (Fig. 2).
Similar to the activity of alirocumab in ODYSSEY LONG TERM, evolocumab indicated CV protection in the OSLER 1 and 2 studies presented at this year’s ACC. The New England Journal of Medicine published both sets of data online simultaneously during the ACC (March 15; ePub). OSLER 1 and 2 are open-label extension studies that randomized 4465 participants in previously-completed phase 2 and 3 trials to remain on the PCSK9 inhibitor with standard therapy or receive standard therapy alone. In pooled data, evolocumab reduced LDL-C by 61% relative to standard of care alone.
Over 78 weeks in ODYSSEY LONG TERM, 3.3% of those randomized to placebo had a CV event (coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal stroke, or unstable angina requiring hospitalization) versus 1.7% of those randomized to alirocumab. This yielded a hazard ratio (HR) of 0.52 or a 48% risk reduction (P=0.02) for alirocumab relative to placebo. In the OSLER studies, 2.18% of those on standard of care alone versus 0.95% of those on the PCSK9 inhibitor had one of a larger list of events (including transient ischemic attacks, revascularization and heart failure leading to hospitalization) at the end of 52 weeks of follow-up for similar relative protection (HR 0.47; P=0.003) (Fig. 3).
Convenience, Patient Adherence and Safety
The PCSK9 inhibitors are proving versatile. For alirocumab, the ODYSSEY CHOICE I (enrolling familial hypercholesterolemia patients) and ODYSSEY CHOICE II (enrolling statin-intolerant patients) found doses ranging from 75 mg to 150 mg administered at intervals of both 2 (Q2) and 4 (Q4) weeks to be effective and well tolerated.
“Both CHOICE I and II looked at the safety and efficacy of Q2-week versus Q4-week dosing. From the patient’s perspective and based on my 5 years of experience with PCSK9 inhibitors, the choice of dosing frequency is definitely an advantage for the patients,” reported Dr. Daniel Gaudet, University of Montreal, Quebec. Dr. Gaudet was a co-author of ODYSSEY CHOICE I and II, which enrolled approximately 1000 patients and found alirocumab, like all other studies conducted so far, to be well tolerated.
“From the patient’s perspective and based on my 5 years of experience with PCSK9 inhibitors, the choice of dosing frequency is definitely an advantage for the patients.”
Due to the large trial programs, safety data with alirocumab are now robust. In pooled data from nine phase 2 and 3 placebo-controlled studies with 3752 patients, the frequency and types of adverse events were “generally comparable to placebo,” reported Dr. Peter H. Jones, Baylor University of Medicine, Houston, TX. In addition, a second set of pooled data that included ODYSSEY LONG TERM found no safety signal, even among those achieving very low LDL-C, as presented by Dr. Jennifer G. Robinson, University of Iowa, Iowa City, IA.
“In one of the largest evaluations ever conducted of patients with pharmacologically induced LDL-C <25 mg/dl [0.65 mmol/L], no specific safety signals were observed,” Dr. Robinson observed. “These are encouraging data in the context of the potential for additional risk reductions with greater LDL-C lowering.”
Additional Slide
Slide 4 (Fig. 4).