Neurology
35th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) 2019
New Long-term Data Supports Early Intensive Therapy in RRMS patients
Stockholm – In a real-world study, patients with relapsing-remitting multiple sclerosis (RRMS) had less clinical disability and longer time to disease progression with an induction versus escalation strategy using a disease-modifying therapy (DMT). The long-term data over a 9-year period in CARE-MS II follow-up confirmed the efficacy of DMT on relapse, disability, and magnetic resonance imaging (MRI) outcomes, and no new safety signals were observed.
On the basis of results from the 9-year CARE-MS II core study and CARE-MS and TOPAZ extension studies, RRMS patients showed declining adverse events (AEs) and stable or improving clinical and radiographic picture. Long-term safety data indicated reduced AEs in years 3-9 compared with years 1-2. Also presented at this year’s ECTRIMS, a real-world study which compared the proportion of patients experiencing disease activity between patients who received the DMT alemtuzumab as a first-line treatment and those who received this agent as escalation therapy over a mean of 38 months. Patients fared better with an induction therapy strategy, including having higher odds of remaining free of disease activity and lower risk of EDSS worsening.
For an exclusive interview with Dr. Anthony Traboulsee on the impact to clinical practice, click here
Real-world Data Track Induction Versus Escalation Therapy in RRMS
A United Kingdom-based retrospective study comparing induction to escalation therapy using alemtuzumab tracked efficacy and safety among RRMS patients.
“Early aggressive therapeutic suppression of inflammatory activity associates with better outcome.”
The study, presented by Dr. Eleanora Rigoni, Foundation C. Mondino National Neurological Institute, University of Pavia, Italy, reviewed data from 124 RRMS patients, each of whom received just two courses of alemtuzumab. Twenty-five patients (20%) had received no prior MS treatment before receiving alemtuzumab, whereas 99 patients (80%) received alemtuzumab as escalation therapy, after receiving other DMTs.
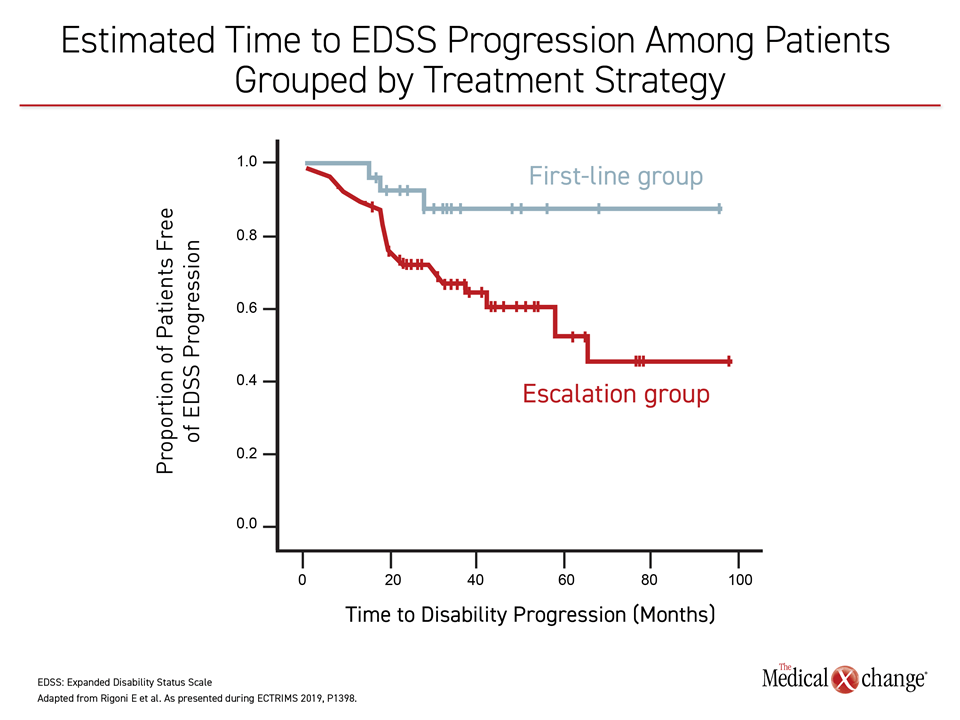
Though radiographic evidence of disease was similar between the induction and the escalation groups, significantly more patients in the induction group presented no evidence of disease activity (NEDA-3; 72% versus 48%, P=0.03). Furthermore, patients who had alemtuzumab induction were more likely to have a longer time to EDSS progression, compared with those receiving escalation therapy (Figure 1).
Baseline differences between the induction and escalation groups were observed, including a lower mean EDSS score for the induction group (3 vs. 4, P=0.05). Mean disease duration was longer among the escalation group (4.2 vs. 9.3 years, P=0.019) and the mean age at first course of alemtuzumab was older for the escalation group than the induction group (43 years vs. 38 years, respectively, P=0.05).
“We confirmed in the real-world setting that, compared to escalation, …induction with alemtuzumab is associated with higher chances of remaining free of disease activity,” noted Dr. Rigoni and colleagues.
CARE-MS II: Long-Term Follow-up Tracks DMT Use over Time
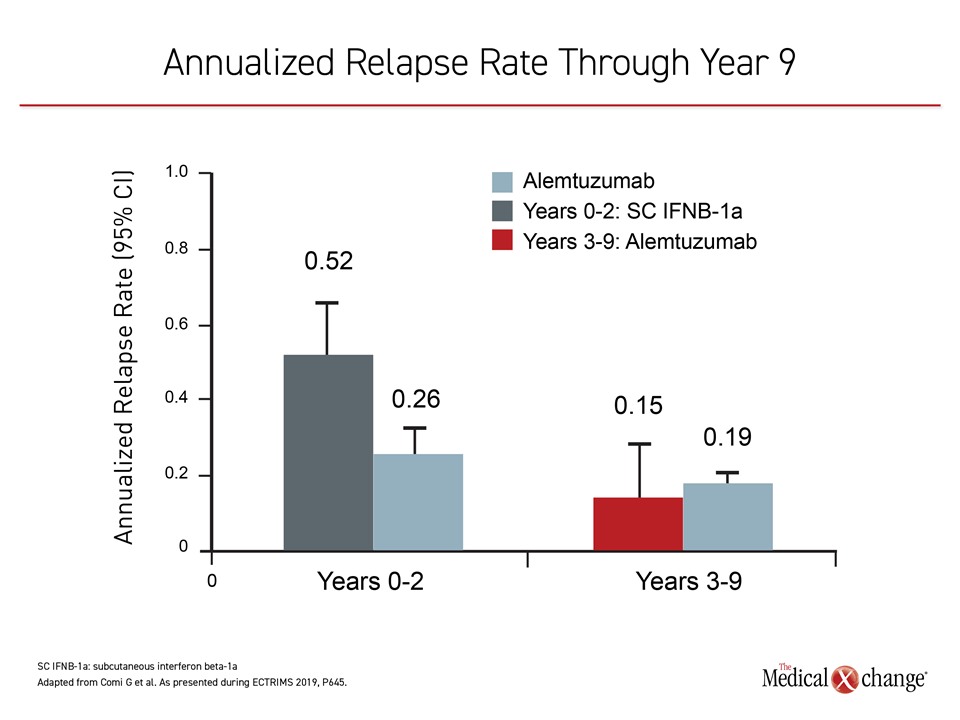
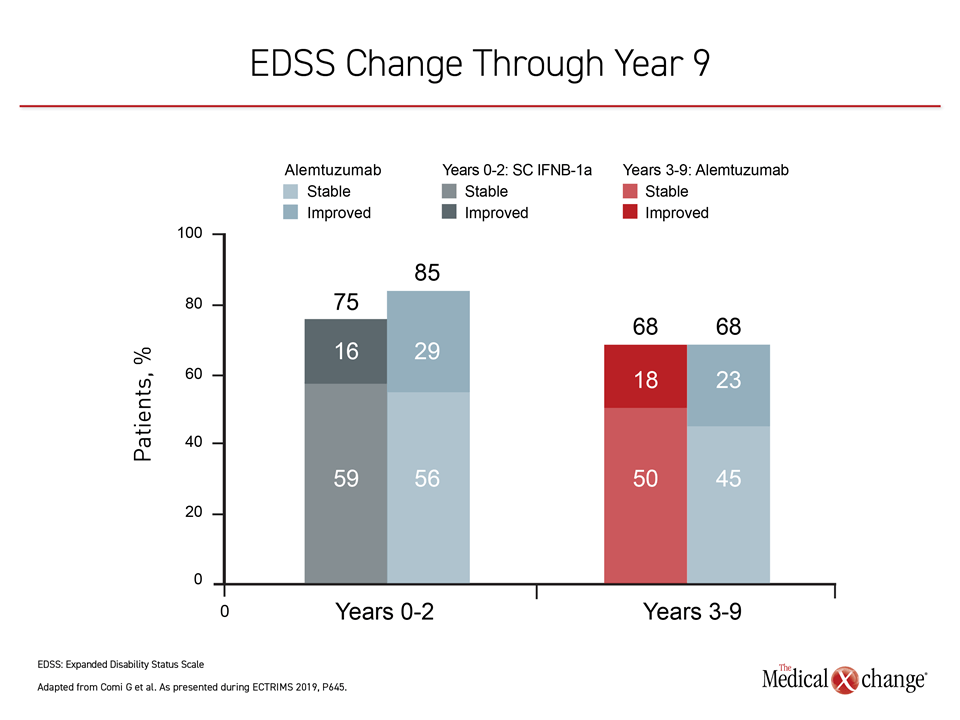
Of the 435 original CARE-MS II patients, 393 entered the extension studies; two-thirds of the original cohort (n=288) completed the full 9-year extension studies CARE-MS and TOPAZ. In patients with active RRMS receiving alemtuzumab, 68% saw improvement or stability in their EDSS scores at year 9, with an annualized relapse rate of less than 20%, reported Dr. Giancarlo Comi, University Vita-Salute San Raffaele, Milan, Italy, and co-investigators (Figure 2).
Alemtuzumab, initially delivered via two infusion courses 12 months apart, was re-dosed if needed in the extension period. Although other DMTs were permitted, 41% of patients received only 2 courses of alemtuzumab and no other DMT through to year 9, noted Dr. Comi.
Low Disease Activity Seen on MRI
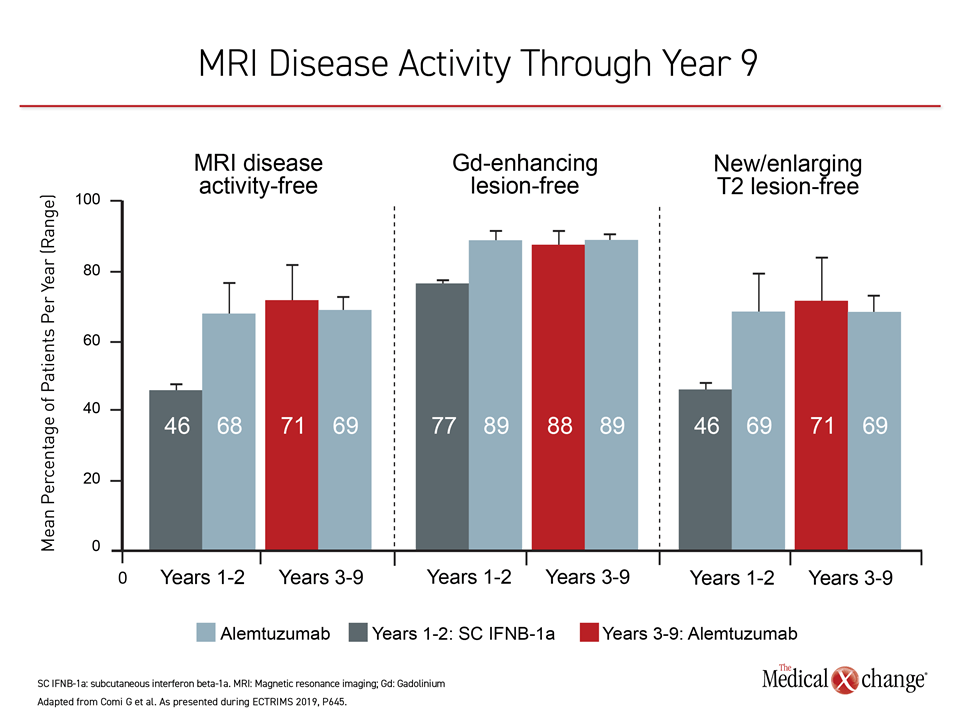
Almost 90% of patients in the extension studies were free of gadolinium-enhancing lesions on MRI at year 9 and 71% were free of new or enlarging T2 lesions and 71% were completely free of MRI disease activity.
“Over two-thirds of patients were completely free of MRI disease activity at year 9.”
The investigators reported that 60% of the study cohort did not report 6-month confirmed disability worsening (CDW), and 49% reported 6-month confirmed disability improvement (CDI). The total mean change in EDSS score for the follow-up cohort was +0.32 from baseline to the end of year 9.
The initial CARE-MS II study found that alemtuzumab significantly improved MS-related disability and had fewer new or expanding lesions on MRI, compared to subcutaneous interferon beta-1a for patients with RRMS.
Declining Adverse Events Reported at the 9-year Mark
“The incidence of [AEs] was reduced in years 3-9 compared with the core study [years 1-2], and declined over time,” reported Dr. Comi. Thyroid-related AEs declined after a peak in year 3, and no immune thrombocytopenia occurred more than 48 months after the last alemtuzumab dose.
Conclusion
Patients receiving DMTs in a real-world study showed less disability and disease progression when alemtuzumab was the first therapy received. The CARE-MS II follow-up study of RRMS patients receiving alemtuzumab showed declining rates of AEs over time and generally sustained efficacy.