Dermatologie
CSU: Expert Review and Commentary from Published Literature
Chronic Spontaneous Urticaria: Control in Primary Care
Werner J Barnard, MD
Assistant Professor, University of Saskatchewan
Regina, Saskatchewan
Urticaria is a relatively common clinical complaint that is driven by activation of skin mast cells. The characteristic wheals, commonly referred to as hives, are typically accompanied by other symptoms involving the skin, such as pruritus and edema. As opposed to transient acute urticaria with known triggers, chronic spontaneous urticaria (CSU), also referred to as chronic idiopathic urticaria, is defined by duration of more than six weeks with an unclear etiology. Treatment for CSU can be initiated at the primary care level when patient history does not suggest alternative causes. To achieve the primary treatment goal of an improved quality of life, the most recent guidelines have simplified treatment into four steps. Antihistamines are employed in the first two steps. The anti-IgE antibody omalizumab is added in step three. Other options are reserved for step four. Symptoms can be controlled with antihistamines alone in more than 50% of patients.
Background
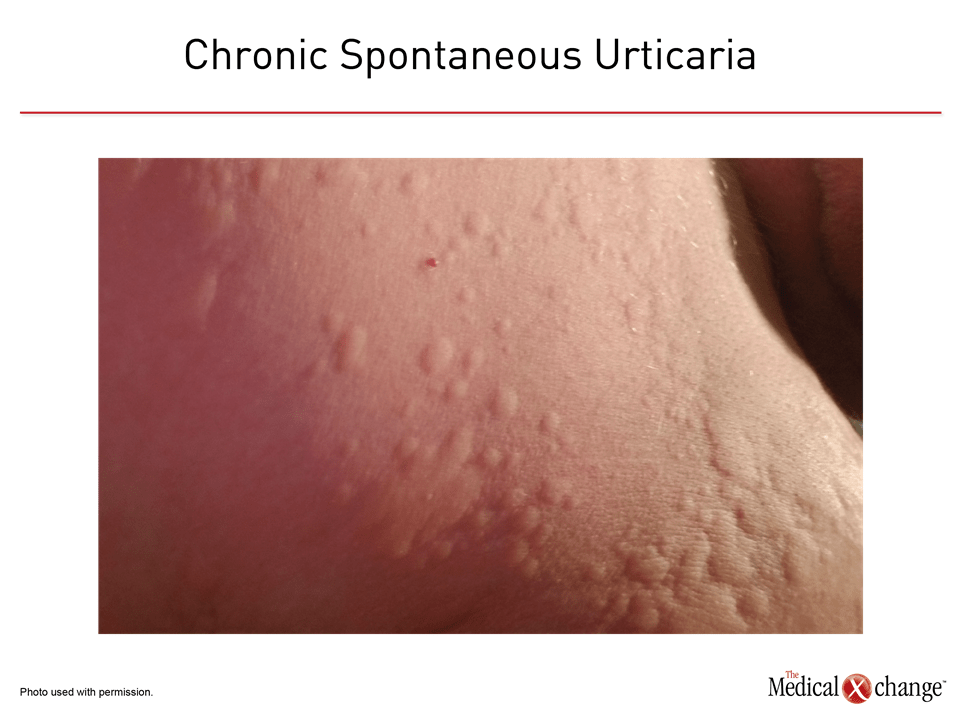
The estimated per-year incidence of CSU in the general population has been in the range of 0.5% to 1.0%,1 making this condition a relatively common complaint (Figure 1). Estimates of the point prevalence rate have been as high as 1%.2 In a survey of Canadian dermatologists, essentially all (98.4%) reported experience treating patients with CSU.3 CSU is observed more frequently in adults than children and in woman than in men.4,5 In a 10-year population-based follow-up of more than one million patients with urticaria, the peak prevalence of CSU was observed between ages 20 and 65 years of age.5 For adults aged 20 years to 44 years, there was a 25% greater hazard ratio for CSU among women relative to men (HR 1.25; 95% CI 1.23 -1.28). Relatively lower numbers of CSU cases were observed in children and individuals older than 65 years of age.
For an exclusive interview with Dr. Werner Barnard on the impact to clinical practice, click here
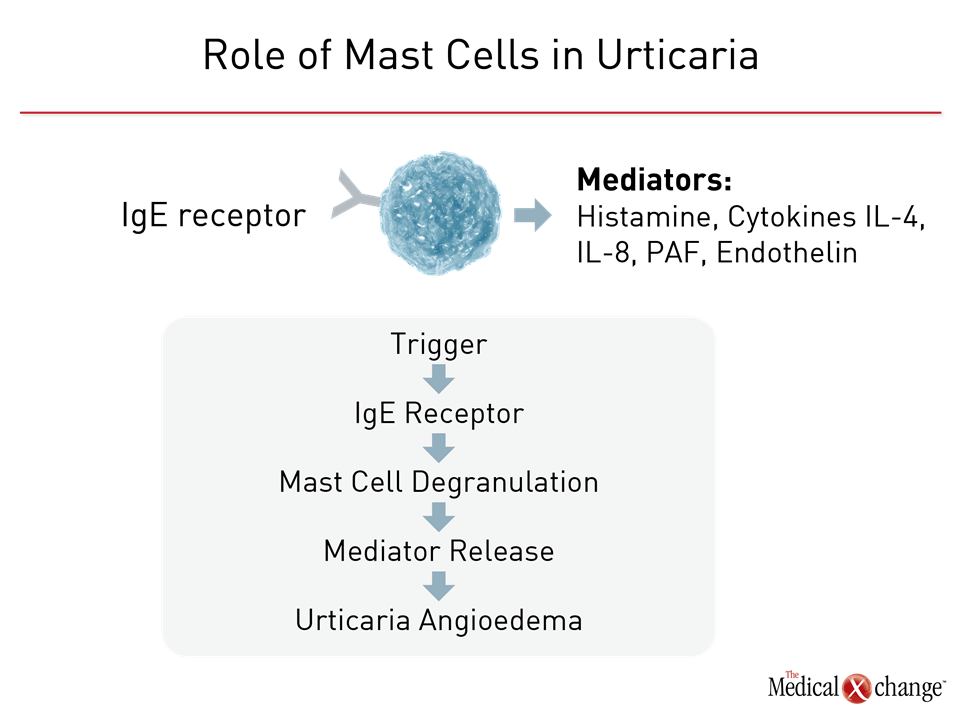
Urticaria of any form, whether acute or chronic, inducible or spontaneous, is typically driven by degranulation of mast cells and basophils in the skin. When IgE receptors on the surface of these cells are triggered, the activated cells release histamine and proinflammatory mediators, such as interleukins-4 (IL-4), IL-5, and IL-8, and platelet activating factor (PAF) (Figure 2).6 The reason for mast cell degranulation in patients with CSU is not yet fully understood. Explanations include an immunomodulatory disturbance involving IgG or autoantigen activity at the IgE receptor.6 Angioedema, which accompanies CSU in up to 50% of patients,7 occurs when mast cell activation occurs deeper or lower in the dermis or subcutis.
In patients with acute urticaria, the list of potential triggers is long and includes food allergies, insect bites, medications, viruses, cold and heat exposure, ultraviolet light exposure, and contact allergens.8 Although CSU is not inducible, patients might associate specific triggers, including specific foods or environmental exposures, with onset or an increased likelihood of an exacerbation. The diagnosis of CSU remains appropriate if urticaria is not reliably or consistently produced upon challenge and symptoms have persisted for longer than six weeks.
Thyroid autoantibodies and IgE receptor antibodies have been observed in patients with CSU, supporting speculation that there is an underlying autoimmune process, but the relevance to clinical management remains undetermined.9 For example, no therapeutic or prognostic value has been associated with the demonstration of these autoantibodies in CSU patients on the basis of an autologous serum skin test (ASST) or autologous plasma skin test (APST).9
CSU is not a life-threatening condition, but it does impose a significant disease burden in objective measures of daily-life functioning when untreated or inadequately controlled.10,11 In an observational study of 673 adult patients with CSU, 299 had moderate to severe activity as measured with patient-completed 7-day urticaria activity score (UAS7),12 but the study demonstrated a substantial disease burden even among those with mild disease. For example, more than one-third of patients with mild disease reported anxiety and or depression, and 25% reported sleep problems. In those with more significant symptoms, the majority reported both of these complaints as well as interference with daily activities and impaired work performance. Perhaps most reflective of the disease burden and the need for effective symptom control, 55% of those with mild disease reported fear or shame about their condition. The proportion with fear or shame exceeded 70% with greater CSU severity.
The course of CSU is variable. In one study, symptoms at one year had resolved in 35% of patients and diminished in nearly half of the remaining.13 In another, which followed patients for five years, the proportion of patients achieving symptom resolution climbed from 30% at one year to 86% at five years, leaving a small but substantial group with persistent symptoms at this time.14
Differential Diagnosis of CSU in Primary Care
CSU, like acute urticaria, appears typically as raised pale lesions, often plaque-like, on an erythematous background. They assume varying shapes and sizes and can appear on any part of the skin.8 CSU lesions, whether or not accompanied by angioedema, often develop within minutes and then dissipate in most cases within 24 hours even as new lesions form. The lesions are not associated with burning or bruising, which suggests vasculitis. When angioedema accompanies CSU, it is most often observed in the face, the genitalia, and the extremities. Angioedema may be painful.
Current guidelines describe the workup of CSU as a rule-out process of alternative diagnoses with shared symptoms.15 Extensive screening and testing for alternative diagnoses in the absence of signs and symptoms of a disease other than CSU is expressly discouraged. Basic laboratory tests, such as a complete blood count (CBC) with differential and sedimentation rate, are reasonable to rule out inflammatory diseases, but it is important to recognize that CSU symptoms are confined to the skin. A thorough history and physical examination that does not raise concern about an underlying systemic pathology is sufficient for a presumptive diagnosis of CSU.
For example, the urticaria associated with Schnitzler syndrome, which, like CSU, often develops in middle age, is accompanied by other characteristic complaints, such as bone pain, weight loss, fatigue, and an enlarged spleen not consistent with CSU.16 The features of cryopyrin-associated periodic syndrome (CAPS), a relatively rare inherited disorder, also features urticaria, but other symptoms, such as fever and arthralgia, will also be present.17
Autoimmune diseases, such as systemic lupus erythematosus and dermatomyositis, can be included in a differential diagnosis of CSU, but, again, serologic testing for these disorders should not be performed in the absence of symptoms beyond urticaria.9 Diagnostic studies to rule out malignancies are not warranted without evidence, such as unexplained weight loss, that would encourage this direction of evaluation. Testing for thyroid antibodies, even if identified, is not useful for guiding management in isolation of other evidence of thyroid dysfunction.
Concomitant angioedema does not alter diagnostic testing even if it expands the list of conditions to be considered in a differential diagnosis. For example, history and physical examination is usually sufficient to rule out hereditary angioedema (HAE), which does not typically present with wheals and has a more severe presentation than the angioedema associated with CSU.15
According to current guidelines, the initial history in patients suspected of CSU should include documentation of onset and course. It should also be conducted to elicit information about any potential triggers associated with onset, such as stress, infections, or medications.15 Validated tools to document disease activity and impact on daily function can be helpful for establishing severity and monitoring changes with initiation of therapy. Validated instruments identified in the guidelines include the patient-completed 7-day urticaria activity score (UAS7),18 which captures both pruritus severity and wheal counts, the angioedema activity score (AAS),19 and the urticaria control test (UCT).20
Evidence-Based Care
The goal of CSU treatment is improvement in quality of life, which is best achieved by complete resolution of symptoms. For CSU, which is defined as idiopathic, treatment is largely dependent on pharmacologic therapies provided within the principle of using as much treatment as needed but as little as possible to achieve the treatment goal.15 Although CSU is by definition idiopathic, this type of urticaria is not always readily distinguished from chronic inducible urticaria. Despite negative or inconsistent associations on provocation testing, patients might associate onset or flares with specific foods, exposures, or activities. In such patients, it is reasonable to encourage patients to avoid their purported triggers independent of pharmacologic therapy.
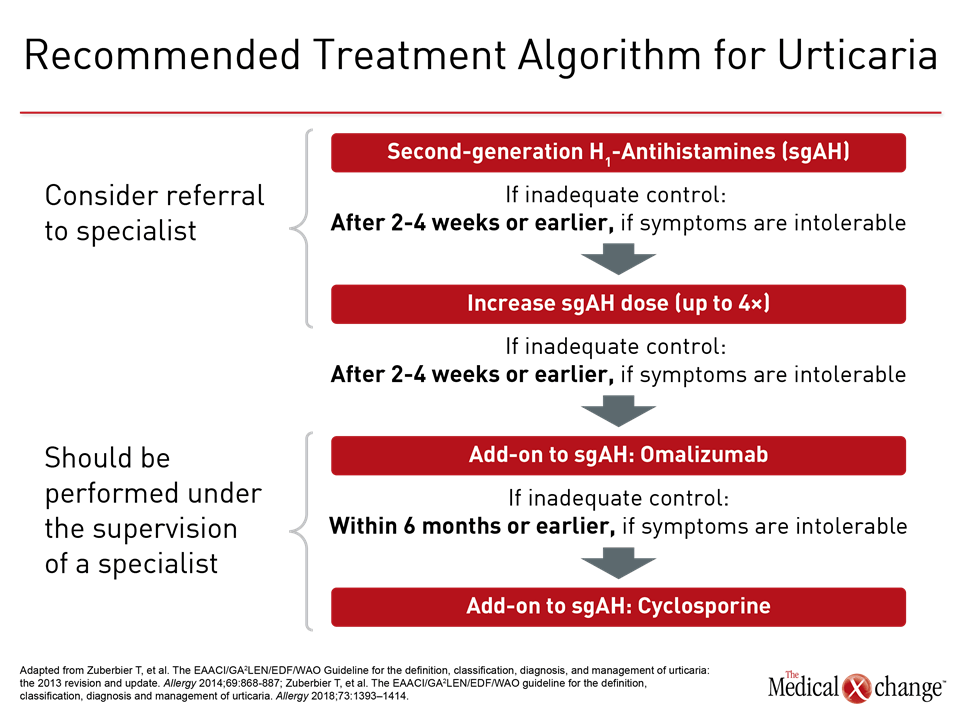
Histamine is the key mediator of urticaria, and antihistamines are the mainstay of therapy (Figure 3). In a randomized trial, continuous therapy was found superior for improving quality of life when compared to as-needed (PRN) treatment in CSU.21 First generation antihistamines are not recommended in the most recent guidelines due to anticholinergic and sedative effects.22 Not all second-generation antihistamines have been tested specifically for urticaria, but there are no comparative studies for those that have, such as loratadine, desloratadine and cetirizine, leading authors of the guidelines to permit considerations of any of the modern agents with low potential for drug-drug interactions.
In those not adequately controlled on continuous dosing of the initially selected second-generation antihistamine, a trial of an alternative agent is appropriate, but combining different agents is not recommended. Rather, the next step in therapy when a single agent does not provide adequate symptom control is an increase in dose. In a double-blind study cited in the guidelines, a two-times higher dose of cetirizine produced significant reductions in pruritus (P=0.006), erythema (P=0.033), and wheals (P=0.015) when compared to a standard dose in cholinergic urticaria.23 Doses of up to four times the standard can be considered, but higher doses than this are not recommended. If symptoms are not controlled within two to four weeks, a switch to a higher dose should be considered. An earlier switch is appropriate if symptoms are intolerable.
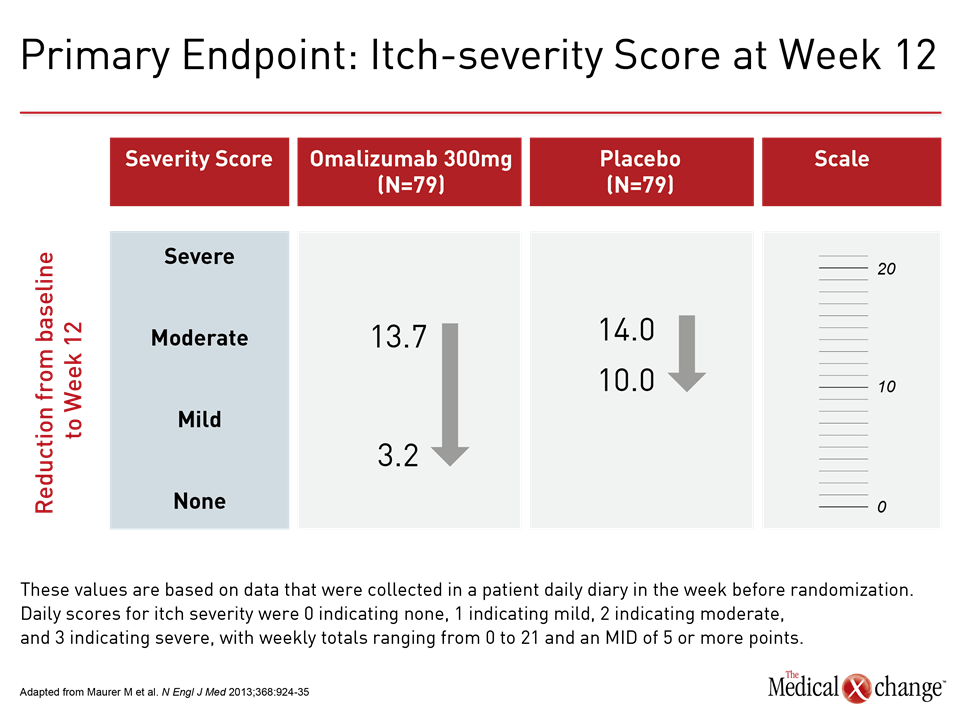
In the absence of adequate control with high-dose second-generation antihistamines, the third step in the guideline algorithm is the addition of the anti-IgE monoclonal antibody omalizumab. By reducing the levels of free IgE and the high-affinity cell surface IgE receptors, omalizumab inhibits key components of mast cell and basophil activation.24 In the phase 3 randomized double-blind registration trial conducted in patients with CSU, the primary endpoint was itch-severity score at week 12. Compared to placebo, the reduction in the median score on the 300 mg dose of omalizumab was more than twice that of placebo (-10.5 vs. -4.0; P<0.001) (Figure 4). This translated into a high degree of relief in most patients and was accompanied by significant reductions in disease activity measured on the secondary endpoints of UAS7 score, weekly hives count, and the Dermatology Life Quality Index.
Omalizumab, which, like placebo, was administered by subcutaneous injection at intervals of four weeks, was well tolerated. The proportion of patients with any adverse event was similar in the 300 mg omalizumab group (65%) relative to placebo (61%). In the group randomized to 300 mg omalizumab, there were no withdrawals due to adverse events versus one withdrawal in the placebo group. No cases of anaphylactic shock or death from any cause occurred in the study. When the 300 mg dose of omalizumab was compared to placebo, the rates of severe adverse events (8% vs. 9%, respectively) and serious adverse events (6% vs. 3%, respectively) were similar.
Initiation of omalizumab should be considered within six months of inadequate symptom control with less intensive therapy, but earlier initiation is acceptable if symptoms are intolerable. The guidelines identify omalizumab, which also reduces angioedema in CSU,25 as effective for improving quality of life and suitable for long-term treatment. The recommended dose is 300 mg omalizumab by subcutaneous injection every four weeks. In the trial, 150 mg was also active, but the 300 mg dose was substantially more effective, similarly well tolerated, and is most commonly used.
Of several options in the fourth guideline-recommended step, cyclosporine, which has a direct effect on mast cell mediator release, is discussed first. Although treatment of CSU is an off-label use, there are placebo-controlled trials demonstrating efficacy.26,27 Cyclosporine is associated with potentially serious adverse events including hypertension, infection, hyperkalemia, and nephrotoxicity. Although these types of adverse events are uncommon at the doses commonly used for CSU, this drug is reserved for those who do not achieve adequate CSU control on the initial guideline-recommended treatments.
Other drugs that have immunosuppressant or anti-inflammatory activity are noted in the guidelines for their potential use in patients not controlled on these four lines of therapy. However, none are explicitly recommended. Glucocorticosteroids, for example, are potentially effective but not appropriate for prolonged use. Case reports suggest benefit in difficult-to-treat CSU from inhibitors of tumor necrosis factor (TNF),28 but an uncertain benefit-to-risk ratio makes this intervention most appropriate for use in specialist centers, according to the guidelines.
Relative to earlier guidelines, the newer recommendations discourage use of sedating first-generation antihistamines and therapies with limited evidence of benefit, such as H2-receptor antagonists and the leukotriene inhibitor montelukast. It is reasonable to expect most patients to achieve adequate control of CSU with the first three currently recommended lines of therapy, according to the newly revised guidelines. If prolonged symptom control is achieved, a trial of de-escalation is appropriate. Although CSU can persist indefinitely in a very small proportion of patients, symptoms more typically diminish gradually with eventual resolution.
Summary
CSU is not a life-threatening condition but can impose meaningful reduction in quality of life even when present in mild to moderate severity. There are no definitive diagnostic tests for CSU, but the diagnosis can be presumed in patients with characteristic skin lesions and pruritus in the absence of signs of systemic disease. Antihistamines are the first-line therapy. Most patients will achieve adequate symptom control following the current treatment algorithm that includes high doses of second-generation antihistamines and, if needed, an anti-IgE monoclonal antibody. The goal of treatment is complete resolution of symptoms. Over time, CSU resolves in most patients although persistent disease is observed in a minority of individuals.
References
1. Sussman G, Hebert J, Gulliver W, et al. Insights and advances in chronic urticaria: a Canadian perspective. Allergy Asthma Clin Immunol 2015;11:7.
2. Maurer M, Weller K, Bindslev-Jensen C, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA(2)LEN task force report. Allergy 2011;66:317-30.
3. Cheung LY, Lynde CW. Chronic Spontaneous Urticaria (CSU): Canadian Dermatologists’ Perspective. J Cutan Med Surg 2016;20:308-12.
4. Asero R. Sex differences in the pathogenesis of chronic urticaria. J Allergy Clin Immunol 2003;111:425-6.
5. Eun SJ, Lee JY, Kim DY, Yoon HS. Natural course of new-onset urticaria: Results of a 10-year follow-up, nationwide, population-based study. Allergol Int 2019;68:52-8.
6. Jain S. Pathogenesis of chronic urticaria: an overview. Dermatol Res Pract 2014;2014:674709.
7. Champion RH, Roberts SO, Carpenter RG, Roger JH. Urticaria and angio-oedema. A review of 554 patients. Br J Dermatol 1969;81:588-97.
8. Schaefer P. Acute and Chronic Urticaria: Evaluation and Treatment. Am Fam Physician 2017;95:717-24.
9. Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol 2014;133:1270-7.
10. Baiardini I, Braido F, Bindslev-Jensen C, et al. Recommendations for assessing patient-reported outcomes and health-related quality of life in patients with urticaria: a GA(2) LEN taskforce position paper. Allergy 2011;66:840-4.
11. Maurer M, Staubach P, Raap U, et al. H1-antihistamine-refractory chronic spontaneous urticaria: it’s worse than we thought – first results of the multicenter real-life AWARE study. Clin Exp Allergy 2017;47:684-92.
12. Maurer M, Abuzakouk M, Berard F, et al. The burden of chronic spontaneous urticaria is substantial: Real-world evidence from ASSURE-CSU. Allergy 2017;72:2005-16.
13. Kozel MM, Mekkes JR, Bossuyt PM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol 2001;45:387-91.
14. Toubi E, Kessel A, Avshovich N, et al. Clinical and laboratory parameters in predicting chronic urticaria duration: a prospective study of 139 patients. Allergy 2004;59:869-73.
15. Zuberbier T, Balke M, Worm M, Edenharter G, Maurer M. Epidemiology of urticaria: a representative cross-sectional population survey. Clin Exp Dermatol 2010;35:869-73.
16. Davis MDP, van der Hilst JCH. Mimickers of Urticaria: Urticarial Vasculitis and Autoinflammatory Diseases. J Allergy Clin Immunol Pract 2018;6:1162-70.
17. Keddie S, Parker T, Lachmann HJ, Ginsberg L. Cryopyrin-Associated Periodic Fever Syndrome and the Nervous System. Curr Treat Options Neurol 2018;20:43.
18. Hollis K, Proctor C, McBride D, et al. Comparison of Urticaria Activity Score Over 7 Days (UAS7) Values Obtained from Once-Daily and Twice-Daily Versions: Results from the ASSURE-CSU Study. Am J Clin Dermatol 2018;19:267-74.
19. Weller K, Groffik A, Magerl M, et al. Development and construct validation of the angioedema quality of life questionnaire. Allergy 2012;67:1289-98.
20. Weller K, Groffik A, Church MK, et al. Development and validation of the Urticaria Control Test: a patient-reported outcome instrument for assessing urticaria control. J Allergy Clin Immunol 2014;133:1365-72, 72 e1-6.
21. Grob JJ, Auquier P, Dreyfus I, Ortonne JP. How to prescribe antihistamines for chronic idiopathic urticaria: desloratadine daily vs PRN and quality of life. Allergy 2009;64:605-12.
22. Church MK, Maurer M, Simons FE, et al. Risk of first-generation H(1)-antihistamines: a GA(2)LEN position paper. Allergy 2010;65:459-66.
23. Zuberbier T, Munzberger C, Haustein U, E T. Double-blind crossover study of high-dose cetirizine in cholinergic urticaria. Dermatology 1996;193:324-7.
24. Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J Allergy Clin Immunol 2004;114:527-30.
25. Staubach P, Metz M, Chapman-Rothe N, et al. Effect of omalizumab on angioedema in H1 -antihistamine-resistant chronic spontaneous urticaria patients: results from X-ACT, a randomized controlled trial. Allergy 2016;71:1135-44.
26. Grattan CE, O’Donnell BF, Francis DM, et al. Randomized double-blind study of cyclosporin in chronic ‘idiopathic’ urticaria. Br J Dermatol 2000;143:365-72.
27. Vena GA, Cassano N, Colombo D, Peruzzi E, Pigatto P, Neo ISG. Cyclosporine in chronic idiopathic urticaria: a double-blind, randomized, placebo-controlled trial. J Am Acad Dermatol 2006;55:705-9.
28. Magerl M, Philipp S, Manasterski M, Friedrich M, Maurer M. Successful treatment of delayed pressure urticaria with anti-TNF-alpha. J Allergy Clin Immunol 2007;119:752-4.