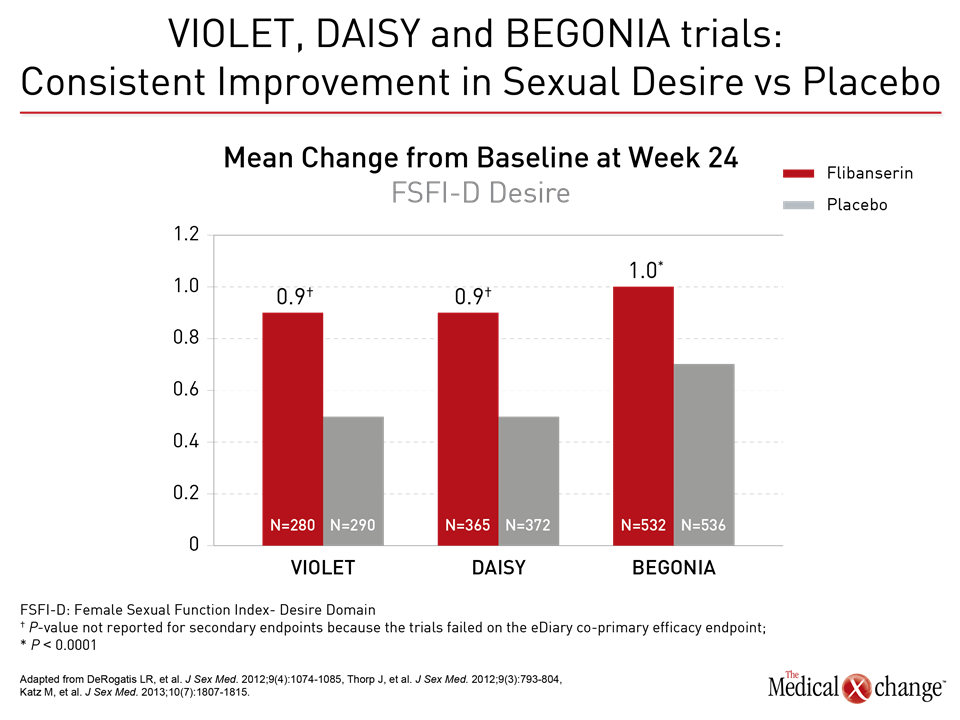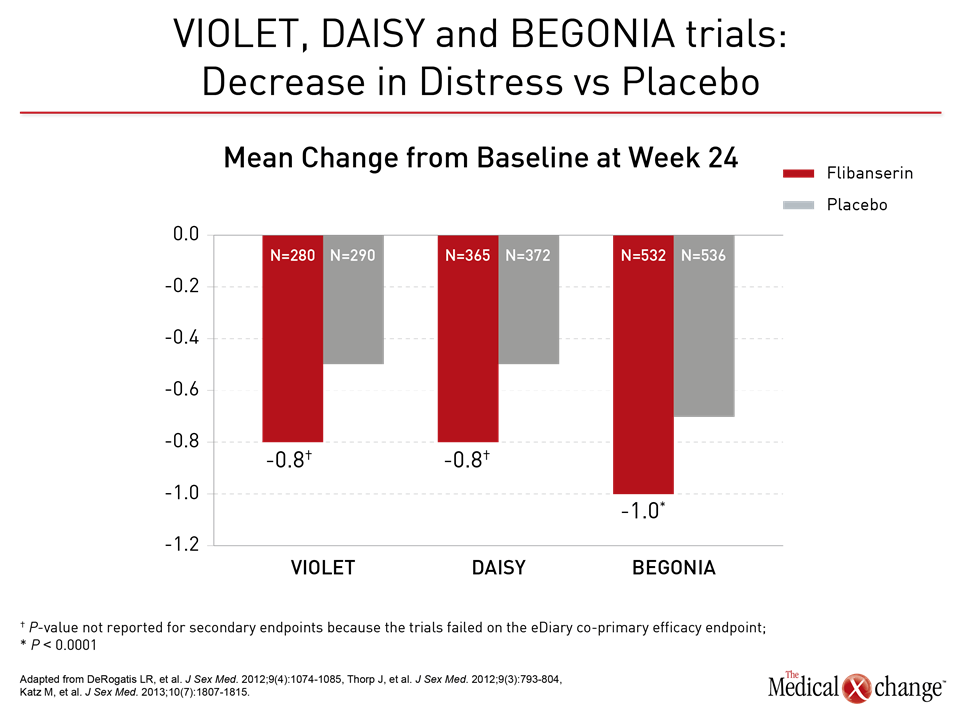Expert Review
Management of Hypoactive Sexual Desire Disorder in Women: Awareness & Treatments
Chapter 3: Treatments – Pharmacology
James G. Pfaus, PhD, IF
Professor, Department of Psychology
Researcher, Center for Studies in Behavioral Neurobiology (CSBN)
Concordia University, Montréal, Québec
Atlanta – Hypoactive sexual desire disorder (HSDD) is estimated to affect about one in every 10 adult women1 . Several promising pharmacotherapies for HSDD are currently in clinical trials as adjuncts to traditional sex therapy. These include hormonal treatments and agents that target the central nervous system (CNS) to increase sexual excitation or decrease sexual inhibition in specific centers of the brain that are crucial for sexual desire. These novel pharmacological agents are new tools in a more comprehensive and patient-oriented approach to the treatment of HSDD.
Introduction
A comprehensive clinical intake interview, along with instruments such as the Decreased Sexual Desire Screener,2 are key in identifying HSDD, a condition that can be treated with psychological interventions, with pharmacological interventions, or with a combination of both. Recently, novel agents are emerging that respond to the neurobiology and mechanisms that lie behind decreased desire. Specifically, therapies that target the CNS and areas of the brain such as the pre-frontal cortex, the locus coeruleus, ventral tegmental area, and medial preoptic area of the hypothalamus, have demonstrated efficacy in the clinical trial setting and at least one therapy that acts on the CNS, flibanserin has been approved by the US Food and Drug Administration and is currently under review at Health Canada.3 Bremelanotide and others still under investigation, all of which act on the CNS and modulate pathways in the brain crucial for sexual desire, are expected to be available in the future for both pre- and post-menopausal women. This will create more choice and a greater ability for clinicians to tailor their strategies to meet the individual needs of all women with HSDD.
History of Pharmacotherapy Use in HSDD
In the absence of any pharmacotherapy indicated to treat HSDD, clinicians have had to use off-label therapies that are not indicated for the treatment of HSDD such as testosterone, a hormonal treatment that has offered benefits but also raised concerns about long-term safety, though to a lesser extent with transdermal delivery.4 Buspirone, a 5-HT1A partial agonist indicated to treat generalized anxiety disorder, has found some success in the management of HSDD5, as has bupropion6, a norepinephrine-dopamine reuptake inhibitor used to treat depression and prescribed for smoking cessation. Both of these off-label therapies are acting on the CNS but neither alone has been studied specifically for the purpose of treating HSDD. Furthermore, there are no safety data specific to the use of bupropion and buspirone in the management of women who have HSDD.
The Basis for CNS-acting Therapies to Treat HSDD
The dilemma of decreased desire is a “network problem” in the brain, according to Dr. Irwin Goldstein, Clinical Professor of Surgery, University of California at San Diego School of Medicine, Director, Sexual Medicine, Alvarado Hospital-San Diego, and Director, San Diego Sexual Medicine, California. The pathways involved in sexual desire feature key excitatory neurotransmitters that are integral to the excitatory systems in the brain including dopamine, melanocortins, oxytocin, vasopressin, and norepinephrine. These transmitters act on neural pathways in the hypothalamus, limbic system, and cortex. In contrast to sexual excitation, the key neurotransmitters involved in the pathways of sexual inhibition include opioids and serotonin (5-HT). The inhibitory systems can dull the excitatory systems so that sexual excitation in response to competent sexual stimuli fails to be activated.1 “We are dealing with poorly-excited and/or highly-inhibited women,” explained Dr. Goldstein, describing patients who have HSDD. “What allows you to be excited is not activated, and what allows you to be inhibited is activated too much. It is a network problem (in the brain) and we can manipulate it to our advantage and help some of our patients.” Images of the brains of women with HSDD and those of healthy controls clearly differ, as evidenced by images captured with functional magnetic resonance imaging in one investigation.7 Women with no history of sexual dysfunction were compared to women with HSDD in terms of their responses to viewing erotic videos, sports, and relaxing images. Women with HSDD had greater activation in the medial frontal gyrus, right inferior front gyrus, and bilateral putamen. Investigators concluded there were differences between women without and those with HSDD in encoding arousal stimuli, retrieval of past erotic experiences, or both.7 “In women who do not have pathology, their brains light up when exposed to sexual stimuli,” said Dr. Goldstein. “Women (with HSDD) will not have things light up (in the brain) at appropriate times.” Furthermore, in another study, where women with HSDD were exposed to two different levels of erotic film segments (high and low) and neutral movies, investigators found women with HSDD experienced diminished left-sided de-activation in the area of the brain that deals with multi-tasking coupled with less right-sided brain activation or more brain de-activation in the medial orbito-frontal cortex.8 “Sex is about turning off the multi-task areas in the brain,” said Dr. Goldstein. “You have to be able to de-activate the things that compete in the brain. You can’t be thinking about the laundry, getting food ready, the children, and finances.”
Pharmacotherapies for HSDD
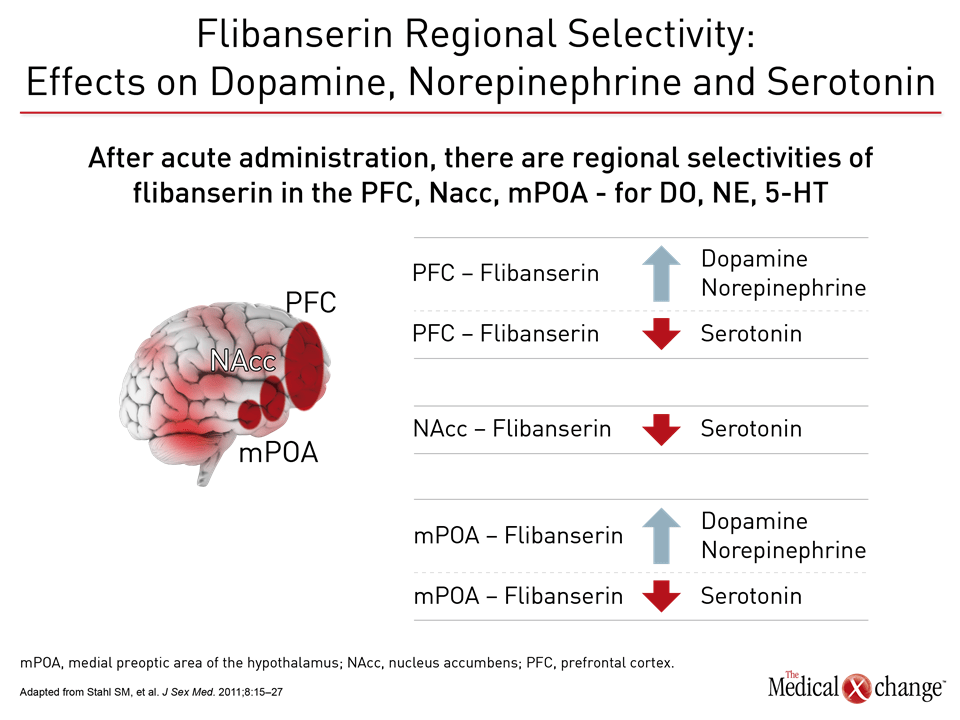
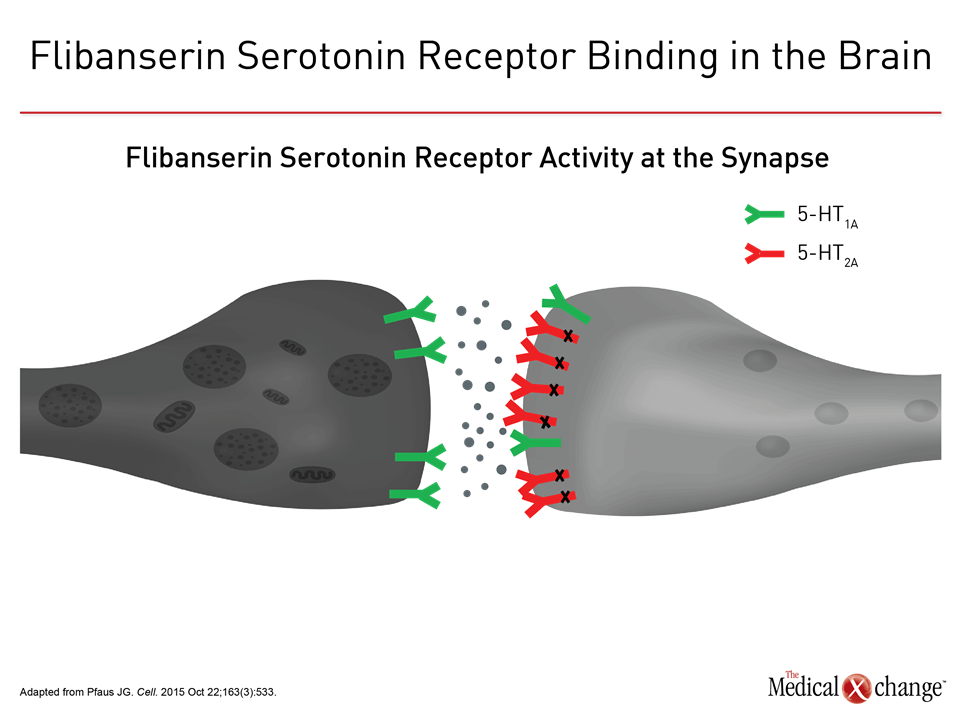
Flibanserin A therapy like flibanserin regulates activation of the prefrontal cortex so that it activates less and therefore does not permit multi-tasking of the brain and distractibility in a sexual encounter, explained Dr. Goldstein. “This is about networks in the brain,” said Dr. Goldstein. “It is not about magic.” (Fig. 1)9 The mechanism of action of flibanserin in the treatment of HSDD is thought to begin with its actions on serotonin receptors in the brain. More specifically, this agent inhibits the release of serotonin by binding to 5-HT1A autoreceptors while simultaneously blocking the post-synaptic action of serotonin on 5-HT2A receptors (Fig. 2).10 Subsequently, these combined actions increase hypothalamic and mesolimbic dopamine transmission, while inhibiting serotonin’s ability to activate sexual inhibition in the prefrontal cortex. Three randomized, double-blind, placebo-controlled trials, known as DAISY, VIOLET and BEGONIA, enrolled premenopausal women and compared the efficacy and safety of flibanserin to placebo. Investigators concluded flibanserin was superior to placebo on numerous measures.11, 12, 13 In the DAISY study, 100mg of flibanserin, taken once daily, produced statistically significant improvements over placebo in satisfying sexual events (SSEs), sexual desire, as measured by the Female Sexual Function Index (FSFI) desire domain score, sexual function, and decreased sexual distress, as measured by Female Sexual Distress Scale-Revised (FSDS-R) total score and FSDS-R Item 13.11 Similarly, in the VIOLET study, 100mg of flibanserin daily produced statistically significant increases in SSEs. FSFI desire domain and total scores increased in a statistically significant fashion, and FSDS-R and FSDS-R Item 13 fell in a statistically significant way.12 Data from the BEGONIA trial were consistent with those from the other two studies: Compared to placebo, flibanserin produced significant improvements in the number of SSEs and sexual desire, as measured by FSFI desire domain score, and decreased distress associated with sexual dysfunction and low sexual desire, specifically on the FSDS-R total score and FSDS-R Item 13 (Fig. 3) and (Fig. 4).13 Flibanserin did not produce any major safety concerns, with documented adverse events including dizziness, nausea, insomnia, somnolence, and anxiety. It is important to note that alcohol is contraindicated with the use of flibanserin because of the risks of hypotension or syncope early in the treatment. A post-hoc analysis of data originating from VIOLET, DAISY, and BEGONIA presented at ISSWSH found the rate of treatment response was 74.4% to 82.2% in premenopausal women with HSDD and that the median time to response was about two months. “It suggests short-term treatment is adequate to know whether it will work or not in patients,” said Dr. James A. Simon, CCD, NCMP, FACOG, Clinical Professor of Reproductive Endocrinology and Infertility, and Obstetrics and Gynecology at the George Washington University School of Medicine, Washington, DC, who presented the post-hoc analysis data at this year’s ISSWSH. “Further, it is recommended that it be discontinued if the patient does not see improvement in her symptoms after eight weeks.” In the real-world setting, however, it would be at the discretion of the patient and the clinician that a patient remains on therapy beyond eight weeks if they are expectant of an onset of response to flibanserin, said Dr. Simon, responding to questions about the data and anecdotal evidence that women have experienced time to response beyond two months. Bremelanotide Another therapy targeting the CNS is bremelanotide, a melanocortin receptor 4-agonist that is administered subcutaneously with an auto-injector on an as-needed basis for HSDD treatment14. The RECONNECT study, which combined two Phase III, multi-center trials, looked at the efficacy and safety of bremelanotide as a treatment for premenopausal women with HSDD. Data from the two trials demonstrated that women who received bremelanotide compared to those given placebo, fared significantly better on endpoints such as the FSFI-Desire, P<0.0001 for both studies, and the Female Sexual Distress Scale-Desire-Arousal/Orgasm Item 13 scores, P<0.0001 for one study and P=0.0007 in the second study. Patients also supplied their own impressions of their experiences with the injectable therapy. Patients were asked to respond to this question, using a 7-point Likert scale: “To what extent do you think you benefited from taking the study drug?” Responders were described as having a self-assessed benefit score of 5 or more. A total of 59% of subjects in the first trial were considered responders, and a total of 58% in the second trial were considered responders. Secondary outcomes were also recorded in the two trials of the RECONNECT study. Dr. Leonard DeRogatis, Director of the Maryland Center for Sexual Health in Baltimore, Maryland, presented data on bremelanotide at ISSWSH, showing that it was linked to significant improvements in FSFI total, arousal, lubrication, orgasm, and satisfaction domain scores, all of which measured P≤0.01.
The Impact of Therapy in a Sample of Patients
A thematic analysis recently presented at ISSWSH of a small sample of women, both premenopausal and postmenopausal, who had been prescribed flibanserin indicated they experienced both sexual and non-sexual changes with the medication, according to Sue Goldstein CCRC, AASECT-CSC, IF, Manager of the Clinical Research Division and Educational Programming at San Diego Sexual Medicine, California. Patients were not asked specific questions, but instead were asked what their experience had been with flibanserin. A total of 23 patients who had been on flibanserin found they experienced improvements in libido, orgasm intensity, as well as initiation of sexual activity and receptivity to sexual activity. Some respondents also identified non-sexual impacts on their lives such as feeling happy and feeling decreased stress and anxiety. “While on the medication, they were feeling like sexual beings and feeling energetic,” noted Goldstein, although she emphasized that the analysis had several limitations, including no specific study protocol and a small number of patients.
HSDD Therapies under Investigation
Other agents are currently being investigated for the management of HSDD in premenopausal women, and are designed to be taken on an on-demand basis. These novel therapies include an agent whose active ingredients are testosterone and the phosphodiesterase inhibitor-5 sildenafil (which is used to treat erectile dysfunction in men).The testosterone component is designed to elevate brain sensitivity to sexual cues, with the sildenafil component designed to increase genital sexual arousal.15 Another agent under study for HSDD contains testosterone and the 5-HT1A agonist buspirone16. It increases the brain’s sensitivity to sexual cues through testosterone but suppresses inhibitory mechanisms in the prefrontal cortex via buspirone. Still another experimental therapy for HSDD is a combination of the anti-anxiety medication trazodone and the anti-depressant medication bupropion.17 The combination is thought to make the movement between states of sexual excitation and inhibition more labile, giving the patient more ability to activate sexual excitation in appropriate and desired circumstances.
Conclusion
For women with HSDD, there are reasons to be optimistic. Indeed, the therapeutic picture looks bright. There is now flibanserin, the commercial availability of bremelanotide is imminent, and other experimental therapies may become tools in the future for clinicians who treat HSDD. Therapies that target the CNS are offering novel strategies for managing HSDD. One of the issues moving forward with the ongoing exploration of pharmacotherapies to treat HSDD is ensuring that patients and clinicians have access to accurate information about mechanism and efficacy, along with more comprehensive treatment strategies, so that patients have the choices they need for the best therapeutic outcome.
References
1. Goldstein I, Kim NN, Clayton AH, et al. Hypoactive Sexual Desire Disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) Expert Consensus Panel Review. Mayo Clin Proc. 2017 Jan;92(1):114-128 2. Clayton AH, Goldfischer ER, Goldstein I, Derogatis L, Lewis-D’Agostino DJ, Pyke R. Validation of the decreased sexual desire screener (DSDS). J Sex Med. 2009 Mar;6(3):730-8 3. Joffe HV, Chang C, Sewell C, et al. FDA Approval of Flibanserin—Treating Hypoactive Sexual Desire Disorder. N Eng J Med. 2016 Jan 14;374(2):101-4. 4. Braunstein GD, Sundwall DA, Katz M, et al. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch Intern Med. 2005 Jul 25;165(14):1582-9. 5. Landen M, Eriksson E, Agren H, Fahlen T. Effect of buspirone on sexual dysfunction in depressed patients treated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1999 Jun;19(3):268-71. 6. Segraves RT, Clayton A, Croft H, Wolf A, Warnock J. Bupropion sustained release for the treatment of hypoactive sexual desire disorder in premenopausal women. J Clin Psychopharmacol. 2004 Jun;24(3):339-42. 7. Arnow BA, Millheiser L, Garrett A, et al. Women with hypoactive sexual desire disorder compared to normal females: a functional magnetic resonance imaging study. Neuroscience. 2009 Jan 23;158(2):484-502. 8. Huynk HK, Beers C, Willemsen A, et al. High-intensity erotic visual stimuli de-activate the primary visual cortex in women. J Sex Med. 2012 Jun;9(6):1579-87. 9. Stahl SM, Sommer B, Allers KA. Multifunctional pharmacology of flibanserin: possible mechanism of therapeutic action in hypoactive sexual desire disorder. J Sex Med. 2011 Jan;8(1):15-27. 10. Pfaus JG. Treatment for hypoactive sexual desire. Cell. 2015 Oct 22;163(3):533. 11. Thorp J, Simon J, Dattani D, et al. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the DAISY study. J Sex Med. 2012 Mar;9(3):793-804. 12. Derogatis LR, Komer L, Katz M, et al. Treatment of hypoactive sexual desire disorder in premenopausal women: efficacy of flibanserin in the VIOLET study. J Sex Med. 2012 Apr;9(4):1074-85. 13. Katz M, DeRogatis LR, Ackerman R, et al. Efficacy of flibanserin in women with hypoactive sexual desire disorder: results from the BEGONIA trial. J Sex Med. 2013 Jul;10(7):1807-15. 14. Clayton AH, Lucas J, DeRogatis LR, Jordan R. Phase I Randomized Placebo-Controlled, Double-Blind Study of the Safety and Tolerability of Bremelanotide Coadministered with Ethanol in Healthy Male and Female Participants. Clin Ther. 2017 Feb 9. 15. Bioemers J, van Rooij K, de Leede L, et al. Single dose sublingual testosterone and oral sildenafil vs. a dual route/dual release fixed dose combination tablet: a pharmacokinetic comparison. Br J Clin Pharmacol. 2016 Jun;81(6):1091-102. 16. van Rooij K, Poels S, Worst P, et al. Efficacy of testosterone combined with a PDE5 inhibitor and testosterone combined with a serotonin (1A) receptor agonist in women with SSRI-induced sexual dysfunction. A preliminary study. Eur J Pharmacol. 2015 Apr 15;753:246-51. 17. Farmer M, Yoon H, Goldstein I. Future Targets for Female Sexual Dysfunction. J Sex Med. 2016 Aug;13(8):1147-65.
Chapter 3: Treatments – Pharmacology
Atlanta – Hypoactive sexual desire disorder (HSDD) is estimated to affect about one in every 10 adult women1 . Several promising pharmacotherapies for HSDD are currently in clinical trials as adjuncts to traditional sex therapy. These include hormonal treatments and agents that target the central nervous system (CNS) to increase sexual excitation or decrease sexual inhibition in specific centers of the brain that are crucial for sexual desire. These novel pharmacological agents are new tools in a more comprehensive and patient-oriented approach to the treatment of HSDD.
Show review