oncology
15th Annual Scientific Meeting of the Society for Neuro-Oncology (SNO)
Addressing the Complex Challenges of Glioblastoma Multiforme
Montreal – Substantial research efforts and resources are being devoted to improving outcomes in newly diagnosed and recurrent GBM. Presentations here indicate standard front-line therapy (radiotherapy and/or temozolomide) is safe and effective in elderly patients, in whom fear of toxicity has often led to limited treatment. Effective strategies for patients who fail existing therapies are sorely needed. Current studies are exploring expansion of the role of temozolomide through dose-dense regimens, the use of bevacizumab, and the role of novel molecular targets.
The list of presentations at this year’s SNO meeting attests that substantial research efforts and resources are being devoted to improving outcomes in newly diagnosed and recurrent glioblastoma multiforme (GBM). Broadly speaking, these endeavours may be categorized as refinements to and improved targeting of existing treatment regimens and development of new agents and combinations. Data emerging in both areas are engendering optimism that within a few years, survival rates in GBM will increase markedly with effective and tolerable therapy that is offered according to specific patient- and tumour-related factors.
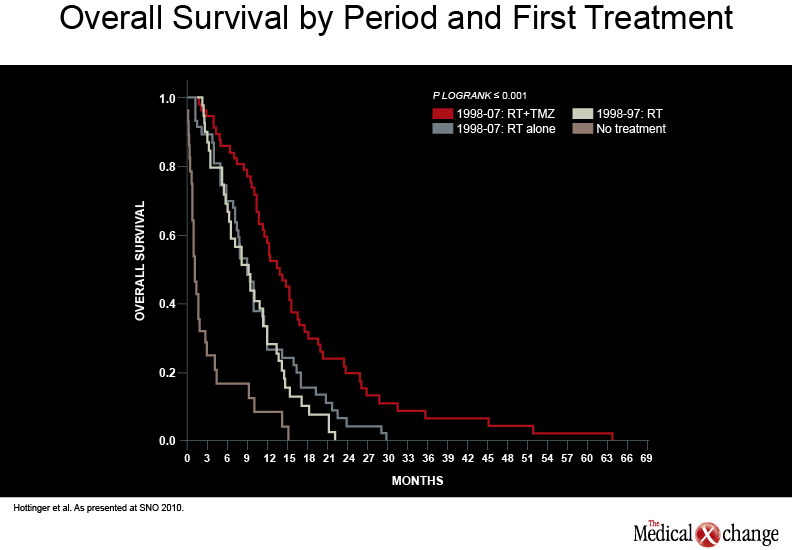
Standard care for patients with GBM involves surgical resection of the tumour to the extent possible, followed by radiotherapy (RT) given concomitantly with daily temozolomide (TMZ, 75mg/m2 x 6 weeks) then adjuvant TMZ (150-200 mg/m2 on 5 days out of 28). In the landmark study by Stupp et al. (N Engl J Med 2005;352(10):987-96), this regimen was shown to extend patients’ median survival by about 37%, to approximately 15 months. Two-year survival in the TMZ group was more than double that of patients in the RT arm (26.5% vs. 10.4%). Data presented at this year’s meeting by Swiss researchers indicated that similar results have been achieved in “real-life” oncology practice (Neuro Oncol 2010;12(4):iv1-iv136, abstract EP-11). For example, 21% of 57 patients treated with chemoradiotherapy vs. 4% of 47 controls receiving RT only survived out to two years (see Graph 1) (Graph 1). A contributing factor, they suggested, is that treating physicians have switched from a mainly palliative to a more aggressive approach to GBM.
Current second-line options for progressive GBM have limited effectiveness. Several factors contribute to this situation, including the challenges of developing agents that bypass the blood-brain barrier and overcome the hurdles of GBM heterogeneity, as well as a complex tumour microenvironment that promotes redundancy and treatment resistance, noted Dr. David Reardon, Associate Deputy Director, Preston Robert Tisch Brain Tumor Center, Duke University, Durham, NC.
Elderly Patients Can Be Treated
Various reports, including retrospective analyses reported here, have indicated that RT +/- TMZ can be safe and effective in older individuals, especially those with reasonable performance status.
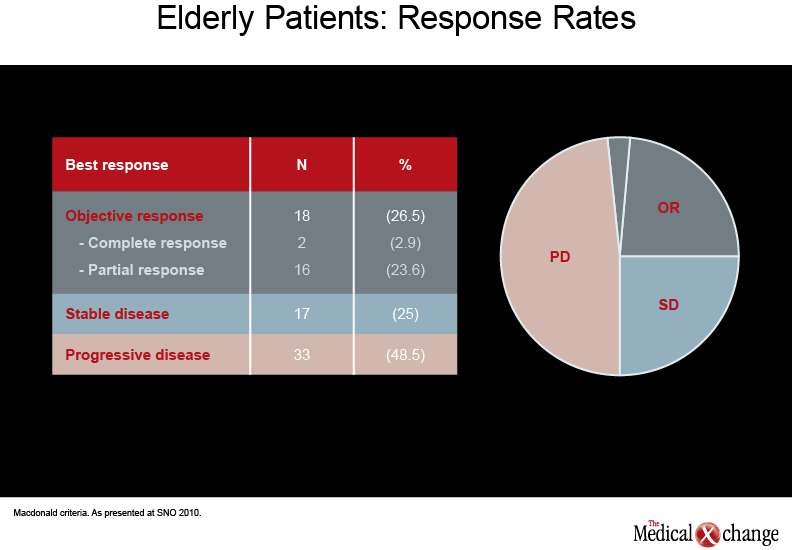
GBM has become significantly more common in elderly patients over the past decade but treatment has often been limited to supportive care in this age group, primarily because of poor expected survival and fear of toxicity, noted Dr. Jaime Gállego Pérez de Larraya, Neurologist, Hôpital La Pitié-Salpêtrière, Paris. Various reports, including retrospective analyses reported here, have indicated that RT +/- TMZ can be safe and effective in older individuals, especially those with reasonable performance status (Neuro Oncol 2010;12(4):iv1-iv136, abstracts RT-21, NO-25). However, information is sparse on elderly patients with poor Karnovsky performance status (KPS). Dr. Gállego Pérez de Larraya and colleagues evaluated the efficacy and safety of TMZ alone (150 to 200 mg/m2/5 days every four weeks, for up to 12 cycles or until progression) in 70 patients aged at least 70 years (Neuro Oncol 2010;12(4):iv1-iv136, abstract OT-28). The majority (91.5%) had undergone biopsy. Two thirds of the patients had KPS 60, while 20% and 16% had KPS 50 and 40, respectively, and 1% had KPS 30. Median progression-free survival (PFS) and overall survival (OS) were 16 and 25 weeks, respectively; 29% of the patients remained progression-free at six months and OS-6 was 44%. An objective response/stable disease was observed in just over half of the patients (see Table 1) (Table 1).
As in other studies, methylguanine methyltransferase DNA repair gene promoter (MGMT) methylation status led to an improved outcome: unmethylated tumours were associated with approximately a doubled risk of death over the course of the trial. It is noteworthy that these results were also accompanied by significant improvements in KPS and quality of life measures, remarked Dr. Gállego Pérez de Larraya.
MGMT and Dose Density
MGMT promoter methylation is widely accepted to suggest better prognosis in patients with GBM receiving RT and TMZ, as it signals reduced expression of the DNA repair enzyme MGMT. MGMT methylation is widely accepted to suggest better prognosis with RT and TMZ, as it signals reduced expression of the DNA repair enzyme MGMT. Most studies involving TMZ now stratify patients according to MGMT methylation status. Work by investigators from the Montreal Neurological Institute (MNI) (Neuro Oncol 2010;12(4):iv1-iv136, abstract CB-44) indicates MGMT promoter methylation is not simply a binary function – i.e., positive or negative – but a continuous variable, explained Dr. Rolando Del Maestro, Professor of Neurosurgery and Oncology at McGill University and Director of the MNI Brain Tumour Research Centre. Considering MGMT promoter methylation as a percentage value may be a more accurate way to predict MGMT expression and activity, he noted. About half of the brain tumour tissue samples in their study of 40 patients had differing MGMT methylation in the four tumour regions evaluated. “It may be preferable to evaluate the tumour total average methylation percentage and to consider the lowest methylated region of the tumour as an indication of its potential response to alkylating agents [TMZ],” the researchers concluded.
Several studies have evaluated whether continuous exposure to TMZ through dose-intense or metronomic TMZ regimens can overcome treatment resistance by depleting MGMT activity. This type of treatment may also have antiangiogenic effects. In the recent RESCUE trial, Perry et al. reported that continuous dose-intense TMZ (50 mg/m2 given daily for 28 days) is active and well tolerated by patients with recurrent GBM after failure of the conventional 150-200 mg/m2 5/28 day regimen. In their study, patients with GBM were prospectively divided into three groups (early [B1], extended [B2], and rechallenge [B3]) according to the timing of progression during adjuvant therapy. Average six-month progression-free survival (PFS6) was 23.9%. One-year survival was 27.3%, 14.8%, and 28.6% for the B1, B2 and B3 groups, respectively (J Clin Oncol 2010;28(12):2051-7).
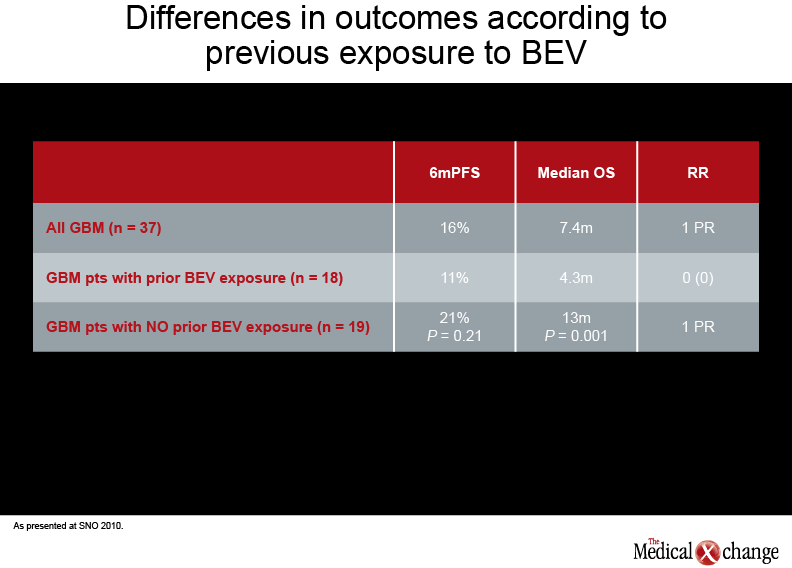
According to interim results from one prospective phase II study of 47 patients with recurrent GBM, TMZ 75-100 mg/m2 on a 21/28 day schedule, given for up to 12 cycles, led to median PFS of 10 weeks and PFS-6 of 23% (Neuro Oncol 2010;12(4):iv1-iv136, abstract OT-09). Median PFS was 10 weeks and median OS 13 months. In another phase II study, reported by Dr. Mustafa Khasraw, Memorial Sloan-Kettering Cancer Center, New York, 37 patients with recurrent or progressive GBM were administered TMZ 50 mg/m2 daily until progression (Neuro Oncol 2010;12(4):iv1-iv136, abstract OT-14). Most had been exposed to bevacizumab (BEV) and other agents in addition to standard chemoradiotherapy with TMZ. The dose-dense study regimen was well tolerated, with the most frequent adverse events being lymphopenia and fatigue. The primary endpoint, PFS6, was 16%; median OS was 7.4 months. Interestingly, in this study, patients who were BEV-naive had significantly better OS and a trend to improved PFS (see Table 2) (Table 2).
“Our interpretation is the patients who have been exposed to BEV, once they have progressed, progress rapidly, while those who were BEV-naive were still able to live longer with other treatments,” said Dr. Khasraw. “Our results highlight the need to stratify patients according to previous BEV exposure, to provide numbers that can be used for future clinical trials.”
Another presentation by MNI investigators posited that neoadjuvant dose-dense TMZ administered presurgically may be an appropriate cytoreductive measure in GBM or other gliomas. In their study, 34 patients chose to receive a daily dose of 75 mg/m2 for 14 days prior to resection, while six patients self-selected to the control cohort. No significant complications such as infection or intraoperative or postoperative hemorrhage were seen with the TMZ. Lymphopenia (3%) was the only grade 3 toxicity observed in the TMZ-treated patients (Neuro Oncol 2010;12(4):iv1-iv136, abstract ST-12).
Although results with dose-dense regimens have been encouraging, Dr. Del Maestro commented, data from the phase-III RTOG-0525 trial (to be reported next year) will be welcome to help clarify the utility of this strategy. “You have to get the methylation in the DNA. If we are using very low levels of TMZ, we can’t measure it there very easily. Do we need a spike or high levels of TMZ to get the level of methylation which is causing the DNA damage? If we don’t get enough TMZ into the cell, it may cause DNA damage but it’s going to be repaired very quickly.”
Targeting Angiogenesis
Glioblastoma is characterized by high levels of microvascular proliferation. Initial clinical trial results with BEV, which inhibits vascular endothelial growth factor (VEGF), were promising: “We saw a very rapid response in grade III and IV patients, and a significant improvement in progression free survival compared with historical benchmarks,” Dr. Reardon indicated. Although there has been concern that VEGF inhibition may simply affect vessel permeability and block contrast uptake, some data indicate BEV has a legitimate antitumour effect. “Fifteen to 20 per cent of those initial study patients had improved MRI scenarios consistent with a durable antitumour effect. [In addition], an important aspect of treatment with VEGF-targeted therapy is that we see clinical improvement in many patients… Some are even able to come off chronic steroid dosing [due to decreased edema, which] has a significant impact on quality of life,” Dr. Reardon remarked. According to Dr. Del Maestro, BEV also prevents radiation-induced injury, which helps ensure patients can continue their full course of therapy.
The ability of BEV to prolong survival is still a subject of study. According to RTOG 0625 results reported by Dr. Gilbert, 117 adult patients with recurrent GBM previously treated with TMZ/radiation were administered BEV 10 mg/kg in combination with either irinotecan 200 mg/m2 every two weeks or dose-dense TMZ (75- 100 mg/m2 for 21 of 28 days) (Neuro Oncol 2010;12(4):iv1-iv136, abstract NO-14). The study suggests that either regimen may be used safely in patients who experience GBM progression after standard care, Dr. Gilbert said. PFS6 and OS were similar in the two arms (see Table 3) (Table 3).
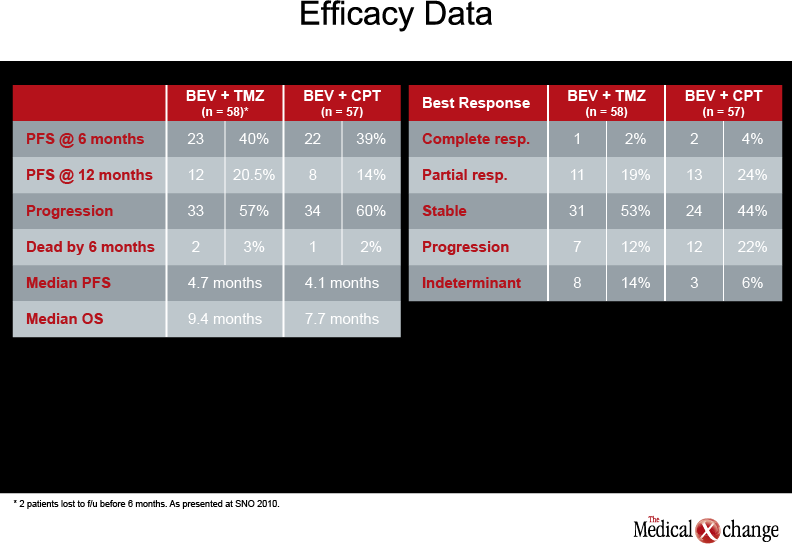
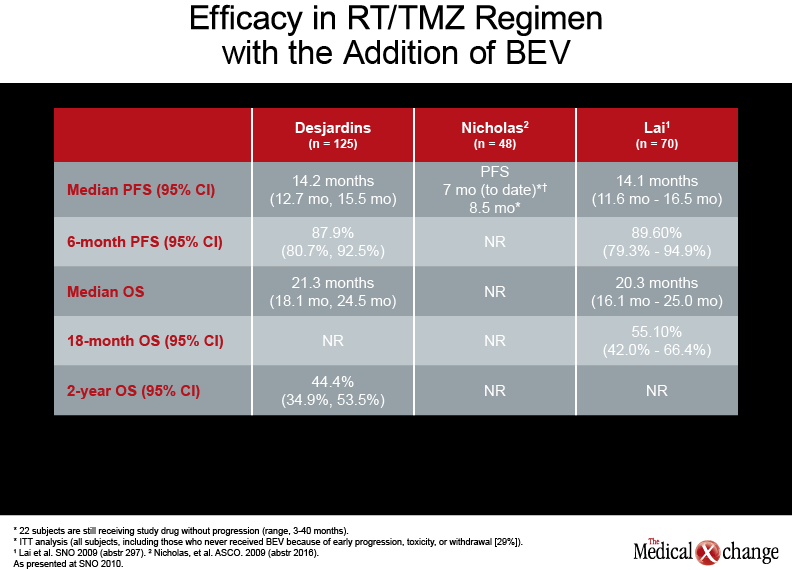
Although there was a trend to better survival in patients receiving the TMZ-containing regimen, this was not the primary endpoint and might be explained by unequal randomization, he cautioned. The use of BEV in addition to standard care in front-line therapy is now being assessed in the phase-III RTOG 0825 trial. A phase II trial reported by Dr. Annick Desjardins, Duke University Medical Center, Durham, South Carolina (Neuro Oncol 2010;12(4):iv1-iv136, abstract OT 34) suggested that the addition of BEV (10 mg/kg every two weeks, started at least 28 days after surgery) to a RT/TMZ regimen followed by BEV, TMZ, and CPT-11 post-RT therapy in newly-diagnosed GBM is well tolerated and improves survival (see Table 4) (Table 4).
According to Dr. Reardon, current data suggest toxicity in this setting is similar to that in the recurrent GBM setting, “although in patients coming out of craniotomy…you have to be very attentive to how the wound is healing before you give [BEV] in order to minimize the risk of wound dehiscence or complications.”
Cause for Optimism
“We need to define patient populations… that will have the best response in combination with the least toxicity…. Hopefully in the future we will be able to better predict patients’ tolerance to therapy based on their personal genomics”
In future, clinical trials and neuro-oncology practitioners will ideally take into account both prognostic variables, which suggest how a patient will fare; and predictive factors, which provide information on the individual’s likelihood of response to or toxicity with a particular therapy, said Dr. Gilbert. “We need to define patient populations… that will have the best response in combination with the least toxicity…. Hopefully in the future we will be able to better predict patients’ tolerance to therapy based on their personal genomics.”
Intense preclinical work is needed to identify the most appropriate of the wide range of novel treatment targets, whether for all patients or for those with specific genetic or tumour profiles, stated Dr. Patrick Wen, Professor of Neurology, Harvard Medical School and Director, Neuro-Oncology, Dana Farber Cancer Institute, Boston, MA. He added that investigators need to explore new ways to assess GBM and its treatment, given the acknowledged limitations of the McDonald criteria in assessing nonenhancing lesions and pseudoprogression/pseudoresponse. Attention to quality of life parameters rather than survival alone is also relevant to trial design.