cardiology
American Heart Association (AHA) Scientific Sessions 2015
SPRINT Trial: Mortality Benefit Associated with Intensive Blood Pressure Control
Orlando – A new therapeutic goal in the management of hypertension has been established by a landmark trial, with highly anticipated findings presented at this year’s AHA meeting. The large multicenter trial, called SPRINT, demonstrated that risk of cardiovascular (CV) events and all-cause mortality are significantly reduced in patients at increased risk if the systolic blood pressure (SBP) treatment goal is 120 mmHg. To bring patients consistently to this goal from the current guideline level of <140 mmHg, it was widely agreed that regimens will have to be selected for their efficacy, convenience, and tolerability.
In SPRINT, the three entry criteria were simple: SBP of 130 mmHg or higher; age of 50 years or above; and one additional CV risk factor. Among the 9,361 patients randomized, the mean SBP at the end of the first year on therapy was 121.4 mmHg in the intensive therapy arm versus 136.2 mmHg for standard therapy. After a median follow-up of 3.26 years, the difference in the rate of CV events was so great in favor of intensive therapy that the data and safety monitoring board (DSMB) terminated the study.
Study Terminated Early for Benefit
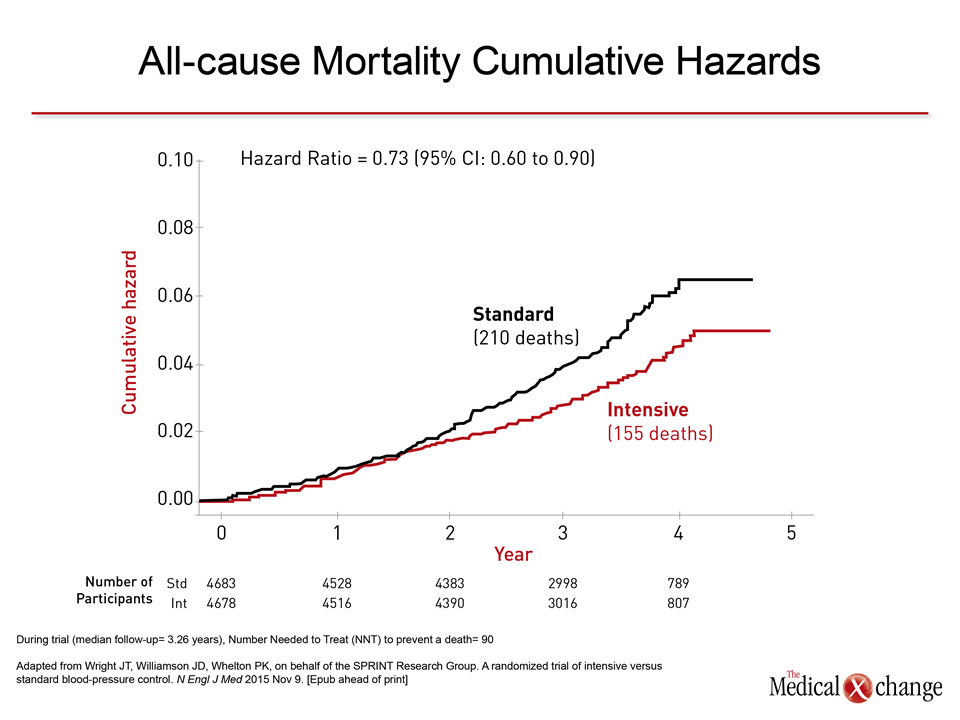
“The event curves separated at about one year and then differences became progressively more dramatic.”
“The event curves separated at about one year and then differences became progressively more dramatic,” reported Dr. Paul K. Whelton, Chairman of the SPRINT Steering Committee and Professor, Department of Epidemiology, Tulane University School of Public Health, New Orleans, LA. In addition to the all-cause mortality reduction of 27% (Fig. 1), there was a 25% reduction in the composite primary endpoint of myocardial infarction (MI), acute coronary syndromes (ACS), stroke, heart failure, or death from cardiovascular causes (HR 0.75; 95% CI 0.64 – 0.89). The relative benefits were consistent across prespecified stratifications, such as relative SBP at baseline above 130 mmHg, gender, and presence or absence of prior CV disease. Of particular note, the risk reduction, although not significantly different, was, if anything, greater in patients aged 75 or older than younger (HR 0.67 vs. 0.80).
Low Risks Outweighed by Benefit
The cost in adverse events was low. Although serious cases of hypotension (2.4% vs. 1.4%; P=0.001), syncope (2.3% vs. 1.7%; P=0.05), and electrolyte abnormalities (3.1% vs. 2.3%; P=0.02), were more common on intensive therapy, the rate of injurious falls was not different (2.2% vs. 2.3%; P=0.71). Adverse changes in kidney dysfunction, such as proportion of patients experiencing a 30% or greater reduction in the estimated glomerular filtration rate was also greater on intensive therapy (1.21% vs. 0.35% per year; P<0.0001), but experts invited by the AHA to comment emphasized that benefits outweighed risks. Tracing the 45-year quest to identify blood pressure targets, Dr. Marc Pfeffer, Professor of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, characterized SPRINT as “a major coup.” Yet, while praising the path SPRINT creates to reduce CV morbidity and mortality, Dr. Pfeffer emphasized that physicians—and their patients—have to develop the will and the fortitude to treat to this new goal. “Don’t expect a thank you from your patients when you add another pill to reduce blood pressure,” cautioned Dr. Pfeffer. He emphasized that physicians have to take the lead in explaining the link between the new goals and improved survival while providing regimens that are effective and convenient.
Average Number of Antihypertensive Drugs: 3
In SPRINT, participating investigators were encouraged but not required to use antihypertensive medications that have previously demonstrated CV risk reductions, such as angiotensin receptor blockers (ARBs). Among diuretics, chlorthalidone, for example, was predominantly used and identified as the preferred agent among thiazide-like agents. In SPRINT, goals were reached with a mean number of 2.8 blood pressure medications in the intensive therapy group versus 1.8 for standard therapy. The likelihood that two or more drugs will be required to achieve the benefits demonstrated in SPRINT emphasizes the importance of considering such strategies as fixed-dose combinations. On the SPRINT trial formulary, azilsartan/chlorthalidone was the only fixed-dose combination with the preferred thiazide-like diuretic. While ARBs overall have been consistently associated with a placebo-like tolerability profile, making them attractive for long-term multidrug hypertension control, azilsartan, and azilsartan/chlorthalidone fixed-dose combination, is an appropriate choice for the SPRINT-defined goals because of the greater efficacy it has shown in head-to-head, double blind trials with other ARBs (Fig. 2). Other strategies that combine simplicity, efficacy, and tolerability will be critical for adherence in treating this asymptomatic condition.
Goals Require Well-tolerated Regimens
Almost 50 years of research support a continuous relationship between rising blood pressure and likelihood of a CV event, according to Dr. Pfeffer. He called the SPRINT trial “a triumph” in redefining the point at which the benefits of intervention exceed the risks. But he said the new goal also creates a “much higher bar” for blood pressure control. While SPRINT provides the evidence “to help our patients live longer,” Dr. Pfeffer indicated that physicians must accept the challenge of identifying the regimens that are adequately potent but simple enough to encourage adherence.
Conclusion
The SPRINT trial has changed the hypertension treatment goals for patients with an increased risk of CV events. For men and women age 50 or older with at least one additional CV risk factor, a reduction in CV events and all-cause mortality was achieved with intensive SBP lowering with a median of only 3 years of follow-up. Importantly, relative protection from intensive blood pressure control was at least as good in those 75 years old and older than those younger.
Additional Slides
Figures 3 (Fig. 3), 4 (Fig. 4), 5 (Fig. 5), 6 (Fig. 6) and 7 (Fig. 7).