Cardiology
Canadian Cardiovascular Congress (CCC) 2017
CCS Guidelines for the Management of Heart Failure: 2017 Comprehensive Update
Vancouver – The optimization of therapy for heart failure (HF) with reduced ejection fraction (HFrEF) will be among areas of emphasis in a soon-to-be-published update of the Canadian guidelines, according to presentations at this year’s annual meeting of the Canadian Cardiovascular Society (CCS). Updates of HF guidelines have been published periodically since 2006, including revisions published last year (Howlett JG et al. Can J. Cardiol 2016;32:296-310), but new HF guidelines are intended to be a comprehensive consolidation of best practice. Of recent changes, particular attention will be paid to survival benefits associated with intensification of treatment in patients with declining left ventricular function.
Building on Triple Therapy in HFrEF
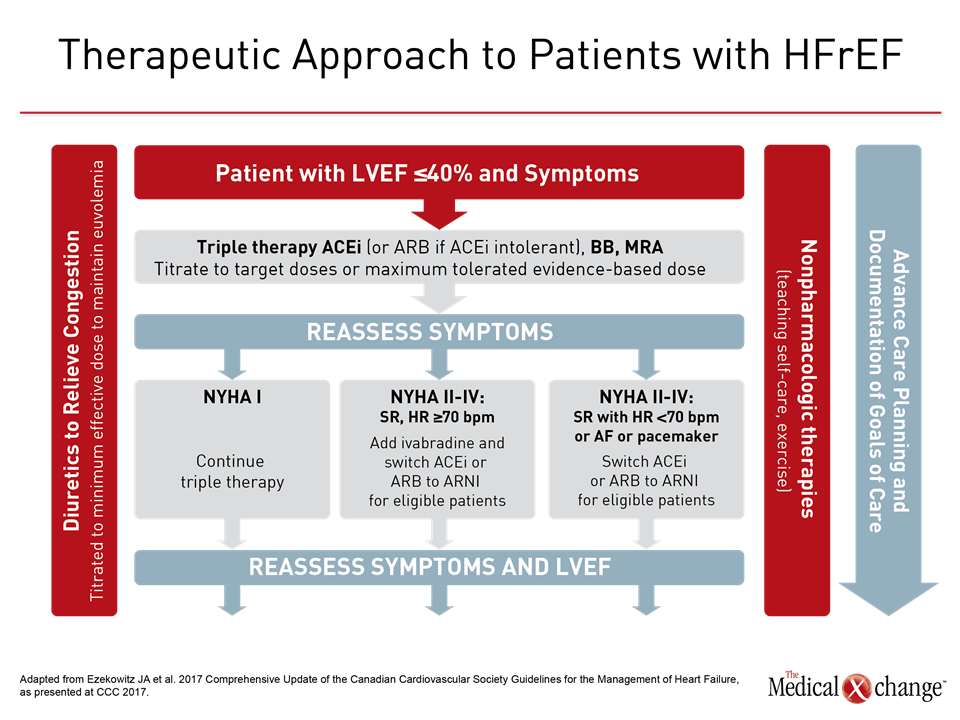
Of strategies to be emphasized in the CCS 2017 update on the management of HF, one will be to build on triple therapy in patients with HFrEF. In patients with advancing HF, standard therapy includes an angiotensin converting-enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) if ACEi intolerant, a beta-blocker, and a mineralocorticoid receptor antagonist. In patients with HFrEF, characterized by a left ejection fraction ≤40% that remain symptomatic, the CCS HF guidelines now recommend additional steps, which include the substitution of sacubitril/valsartan for the ACEi or ARB and, in selected cases, the addition of ivabradine. Although triple therapy should be offered to all patients with HFrEF, intensification of therapy can provide additional benefit as HF advances. These benefits should not be overlooked, according to Dr. Shelley Zieroth, Assistant Professor, University of Manitoba, Winnipeg.
“There are newer agents out there that can, in fact, reduce mortality in your heart failure patients.”
“It is important to remember that according to the MAGGIC (Meta-analysis Global Group in Chronic Heart Failure) score, a patient who is functional New York Heart Association (NYHA) Class II still has a significant one-year and three-year mortality rate, so it is important to not be a victim to physician inertia and care-provider inertia. There are newer agents out there that can, in fact, reduce mortality in your HF patients. We need to think about that rather than thinking everything is just fine,” Dr. Zieroth said.
Considering New Agents
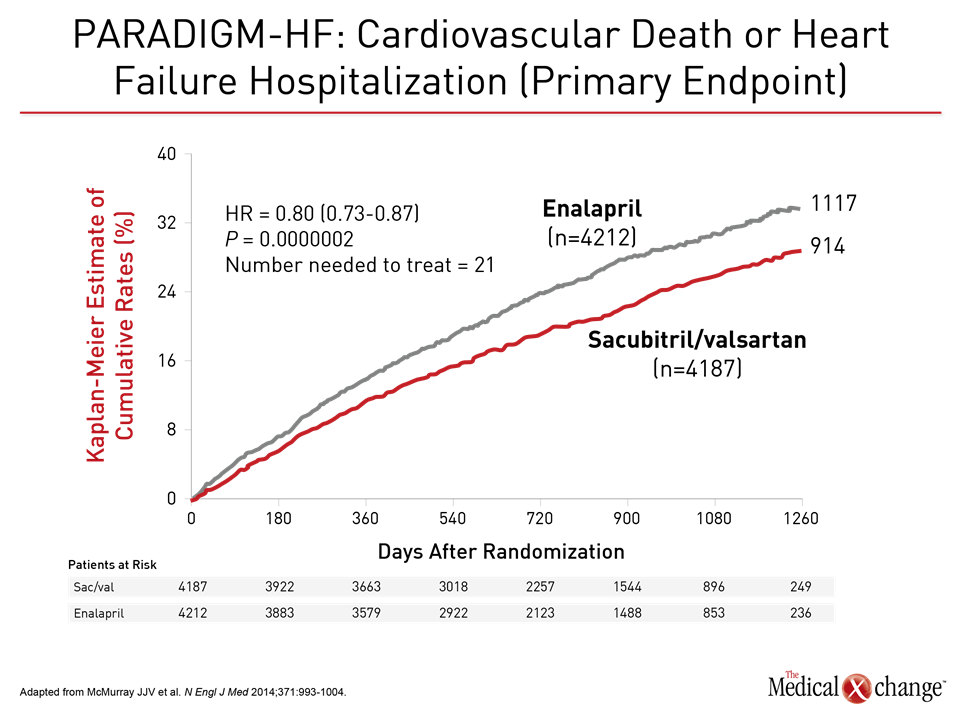
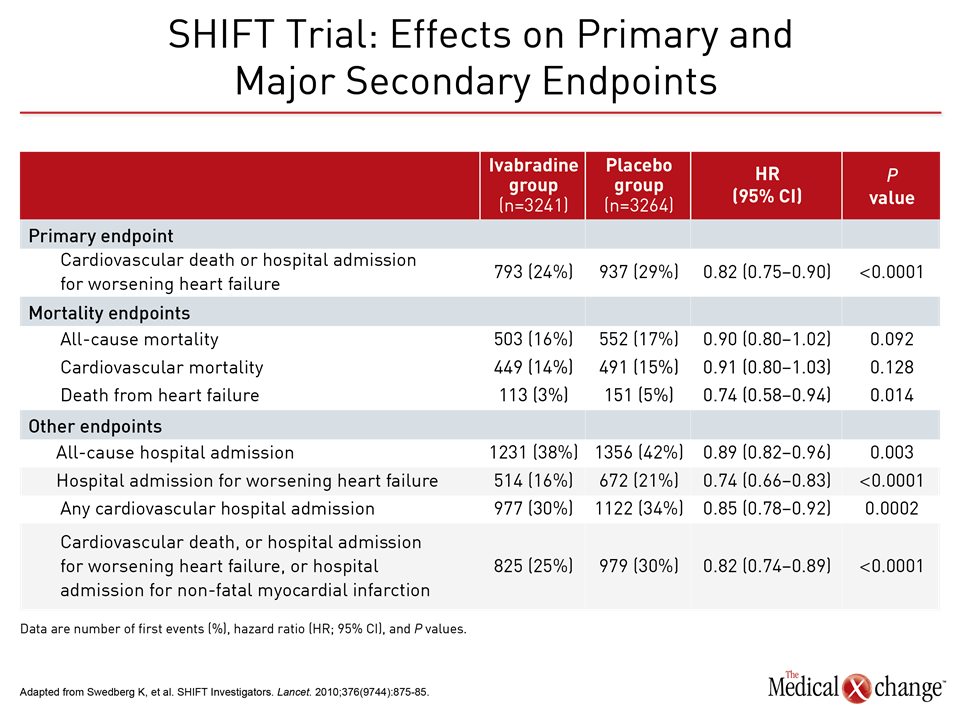
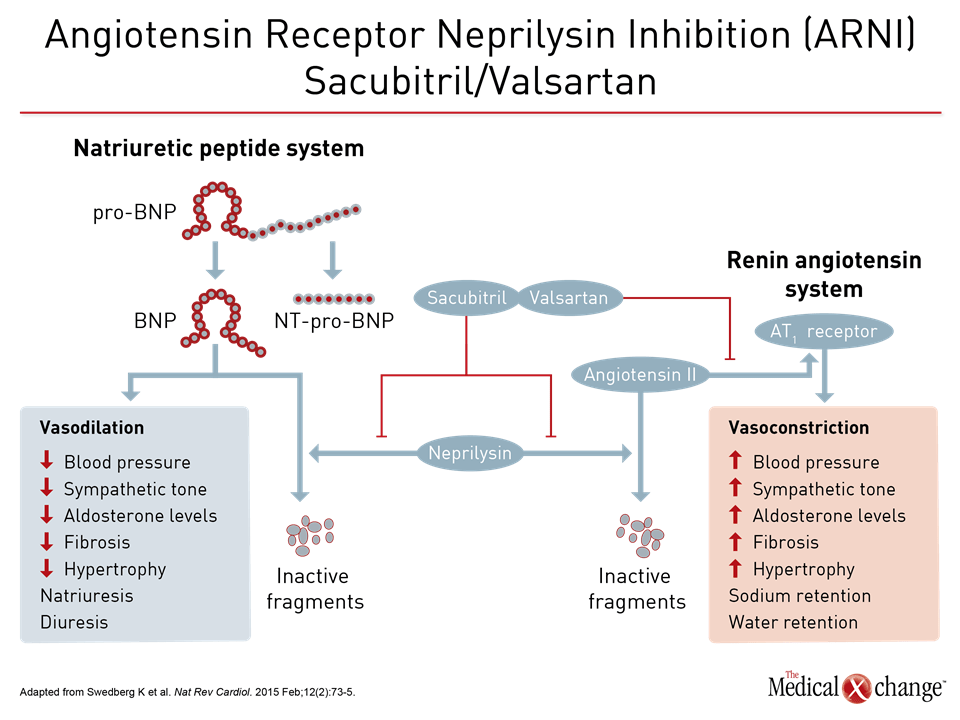
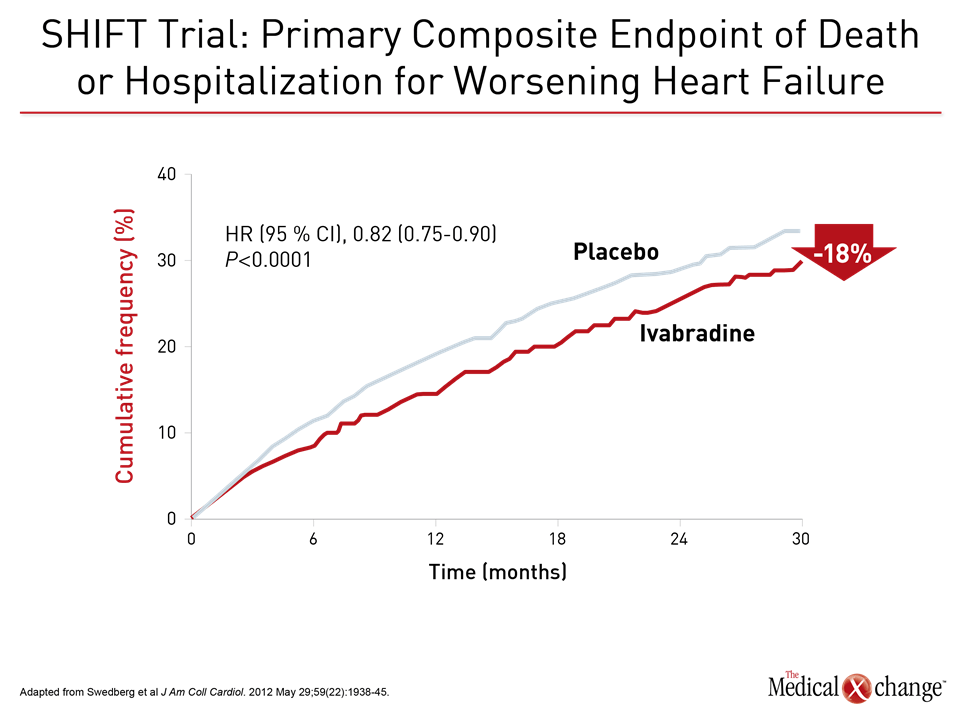
The value of substituting sacubitril/valsartan for the ACEi or ARB in patients with HFrEF is drawn from PARADIGM-HF, which is the largest HF trial ever conducted. The trial was halted early when sacubitril/valsartan was associated with a 20% (P<0.001) decrease in the composite primary outcome of cardiovascular (CV) death or hospital admission for exacerbation of HF compared to enalapril, an ACE inhibitor, in an HFrEF population (Fig. 1). Sacubitril/valsartan is a single agent combining the neprilysin inhibitor with the ARB valsartan. The 2016 CCS guidelines had already recommended this therapy in advancing HFrEF. The comprehensive update includes details based on the clinical evidence about how this therapy is best employed. Of points emphasized in a review at the 2017 CCS meeting, a 36-hour washout period between stopping ACEi and initiating sacubitril/valsartan (100 mg PO BID) is considered mandatory to avoid the development of angioedema. This washout period is not necessary for patients who are already on an ARB. Patients should continue to be monitored for electrolyte levels while on sacubitril/valsartan, as they would be if they were on an ACEI or ARB (Fig. 2). Additional therapies may be appropriate in HFrEF, explained Dr. Zieroth. In particular, ivabradine was given a strong recommendation in the 2016 CCS update that is repeated in the 2017 version. “We have recommended that ivabradine be considered in patients who have a HR >70 bpm, and that is based on the inclusion criteria in the SHIFT trial,” Dr. Zieroth reported. In the Phase III SHIFT trial, ivabradine was associated with an 18% (P<0.0001) decrease in the composite primary outcome of CV death or hospital admission or exacerbation of HF relative to placebo [Swedberg K et al. Lancet 2010;376:875-885] (Fig. 3). Of note, clinicians need to ensure that patients are in sinus rhythm and do not have atrial fibrillation if they opt to prescribe ivabradine to their HF patient, added Dr. Zieroth.
Sub-population Benefits from Novel Agent
Agents that can actually modify the natural history of HF should be instituted as early as possible.
Data from the SHIFT trial demonstrate success in meeting the primary endpoint and even greater success in a sub-population of patients with elevated heart rates, according to Dr. Peter Liu, of the University of Ottawa Heart Institute, Ontario. “If you looked at patients who had heart rates above the median heart rate cut-off of 77 bpm, you can see the endpoint was extremely significant,” said Dr. Peter Liu, noting a 25% reduction in the primary endpoint in that sub-population. “This effect was seen early and was sustained over the trial. This approach can modify the natural history (of the disease) for the patient. Agents that can actually modify the natural history (of HF) should be instituted as early as possible.” If patients continue to be symptomatic and have a reduced ejection fraction after the use of ivabradine and the switch to sacubitril/valsartan, there are further steps with potential benefit. One is an implantable cardioverter defibrillator/cardiac resynchronization therapy. The decision to implant a device should be discussed at length with a patient, according to Dr. Michael MacDonald, University of Toronto, Ontario.
2017 Guidelines also Address Prevention
Closing treatment gaps in patients with advanced HF provides an immediate opportunity to improve outcome, including prolonged survival, but the 2017 guidelines also consolidate recommendations on the full range of issues regarding prevention, diagnosis, and patient monitoring. Several of these issues, including reducing risk of HF in patients with diabetes and the treatment of acute HF were addressed at the 2017 CCS meeting. In patients with type 2 diabetes mellitus (T2DM), tight blood glucose control is essential, as recommended by the Canadian Diabetes Association’s and reiterated in the CCS 2017 HF guidelines update on HF management, but that step alone is not sufficient, according to Dr. Jonathan Howlett, of the Libin Cardiovascular Institute, University of Calgary, Alberta. “There is no convincing evidence that lowering blood glucose, in and of itself, will reduce the risk of HF,” noted Dr. Howlett. However, he said that specific therapies used to control blood glucose levels might have HF benefits, citing studies with sodium-glucose co-transporter 2 (SGLT2) therapies. In the EMPA-REG OUTCOME trial, which compared empagliflozin at two different doses to placebo, the SGLT2 inhibitor was associated with a reduced risk of HF (Zinman B et al. N Engl J Med. 2015;373:2117-2128). Similarly, the CANagliflozin Cardiovascular Assessment Study (CANVAS) in patients with T2DM observed a comparable impact with the SGLT2 inhibitor canagliflozin on mortality due to HF and hospitalization due to HF. But Dr. Howlett added some caveats to the findings: he cautioned that the trials were both not randomized, controlled trials and that the populations in the two trials were poorly characterized with respect to HF and there were low event rates in those populations. Still, the CCS guidelines suggest that the use of these agents be considered for patients with T2DM and established CVD for the prevention of HF-related outcomes.
CCS Guidelines and Acute Heart Failure
The 2017 update on the management of acute HF from the CCS does not contain any specific recommendations with strong evidence for the management of acute HF. Loop diuretics administered intravenously at a dose of 20 to 80 mg/day is suggested. “There is not a good randomized controlled trial (comparing a loop diuretic) against placebo, but they are old, are good and they work,” explained Dr. Justin Ezekowitz, Professor of Medicine, University of Alberta, and Former Chair, CCS HF Guidelines. Adjusting the dose of a diuretic may be needed depending on a patient’s urine output. Similarly, there are scant data from randomized controlled trials to inform the choice of when to discharge a patient with acute HF from hospital, added Dr. Ezekowitz, who is the first author of soon-to-be-published 2017 HF guidelines. The use of continuous positive airway pressure or bi-level positive airway pressure does not support improving outcomes in patients with acute HF but it “does have a role in staving off intubation in some patients,” said Dr. Ezekowitz. When faced with an uncertainty in terms of making a diagnosis of acute HF, ordering a B-type natriuretic peptide test and amino-terminal fragment pro-peptide B-type natriuretic peptide can assist in reducing that uncertainty, according to Dr. Ezekowitz. “In the area of diagnosis, we have a lot of good information about how to get an early, accurate diagnosis using biomarkers to supplement that information. They have a lot of value in the uncertain cases.”
Value of Biomarkers in Improving the Function of HF Patients
“Biomarkers can help us identify co-morbid conditions that need to be better treated such as chronic kidney disease,” said Dr. Eileen O’Meara, Montreal Heart Institute, Quebec. Biomarkers can be common and include physical signs as well as indicators like glucose status, thyroid status, or iron status, and they can be novel, such as natriuretic peptides (NPs). While prognostic scores offer information about mortality and HF hospitalization, they do not reveal information about functional capacity, which is information that many HF patients are seeking. “Prognostic scores do not focus on functional capacity,” noted Dr. O’Meara Remote monitoring devices, however, can play a role in determining functional capacity. The CardioMEMS, for instance, which was FDA-approved in 2015 and which provides pressure readings of the pulmonary artery in HF patients, was found to improve exercise capacity and quality of life in HF patients, according to data presented at the Annual Meeting of the American College of Cardiology in 2016. Data from the PARADIGM-HF trial indicate that elevated circulating levels of NPs predict unfavourable outcomes in HF while a reduction in NP levels is linked to a better patient prognosis. In the PARADIGM-HF trial, patients who were able to achieve an NT-proBNP level <1,000 pg/ml after one month of randomization experienced a decreased risk in the primary endpoint (Fig. 4). Looking to the future, it may be that biomarkers inform the choices of therapies that are selected to manage HF, noted Dr. O’Meara.
Treating Patients with LVEF Rates >40 and <50%
“In the analyses that I have seen, (it) suggests that the mid-range ejection fraction tends to behave more like the reduced ejection fraction group than the preserved ejection fraction,” commented Dr. Howlett. Dr. Karl Swedberg, Senior Professor of Medicine, Department of Molecular and Clinical Medicine, Sahlgrenska Academy, University of Gothenburg, Sweden, and Professor of Cardiology, National Heart and Lung Institute, Imperial College, London, made the same point. “These patients are more similar to the HFrEF population (than patients with preserved ejection fraction) and should be approached with the same type of pharmacological therapy,” said Dr. Swedberg.
Conclusion
Since 2006, there have been substantial advances in the management of heart failure, particularly HFrEF. In the comprehensive 2017 CCS guidelines on HF, an effort has been made to consolidate what is known about best practice regarding prevention of HF, treatment of early disease, and improving survival. Not least important of these recommendations, building on triple therapy with the addition of sacubitril/valsartan and ivabradine when appropriate is one of the evidence-based steps toward improved outcomes.