Rheumatology
2017 ACR/ARHP Annual Meeting
Emerging Options: Moving Toward JAK1 Selectivity in Rheumatoid Arthritis
San Diego – Next-generation JAK inhibitors with improved selectivity for JAK1 are reaching late stages of clinical testing in rheumatoid arthritis (RA). Of the four JAK enzymes, JAK1 is the most closely associated with upregulation of the inflammatory cytokines driving RA. The goal of the selectivity is not only better suppression of these cytokines but also fewer off-target effects. Next generation agents are showing efficacy in RA patients inadequately controlled on conventional synthetic (csDMARD) or biologic disease-modifying antirheumatic drugs (bDMARDs). At the 2017 ACR, data with newer agents included results from phase 3 trials.
New Agents Reaching Regulatory Approval
Building on the clinical utility of tofacitinib, the currently approved JAK inhibitor, a new generation of JAK inhibitors has been engineered to provide greater efficacy and safety. The agents approaching regulatory approval include upadacitinib, baricitinib, and filgotinib. These differ for relative JAK1 selectivity and may not be interchangeable, but the data, including phase 3 data, demonstrate high rates of disease control in patients poorly responsive to other options. Safety data are encouraging. From the six-trial phase 3 study program with upadacitinib called SELECT, two placebo-controlled were presented at this year’s ACR/ARHP meeting. One, called SELECT-BEYOND was a latebreaker and enrolled patients who had failed at least one biologic. The other, SELECT-NEXT, enrolled patients refractory to csDMARDs. Both randomized patients to 15 mg or 30 mg of oral once-daily upadacitinib or placebo. In both studies, response curves favoring active therapy diverged almost immediately.
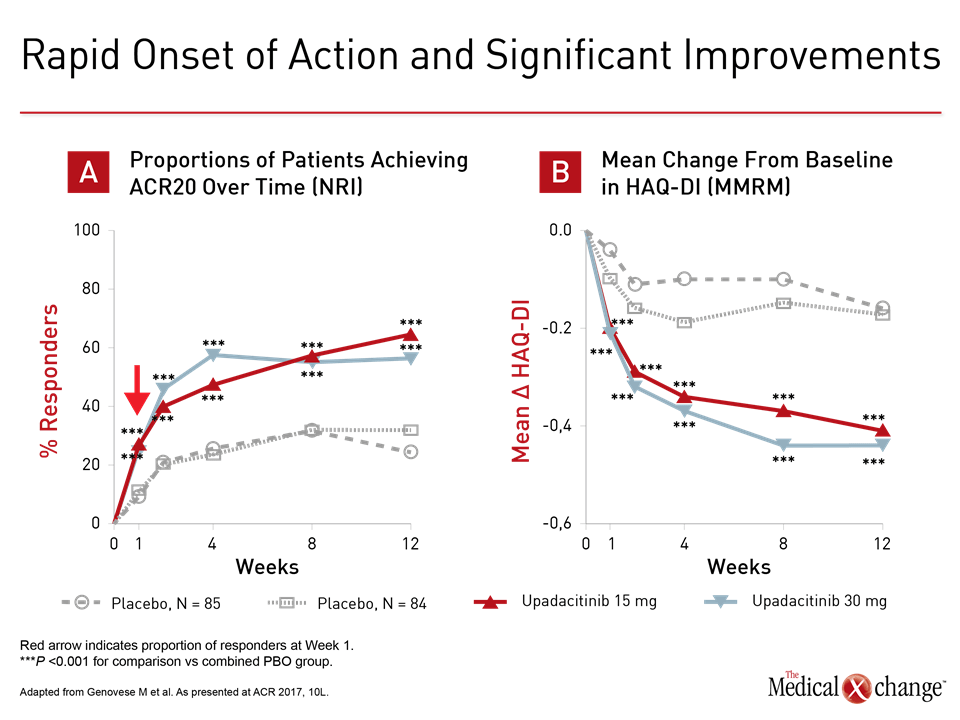
The steep improvement in disease control on multiple measures is consistent with rapid onset of JAK1 selectivity.
“Onset was remarkably rapid with statistically significant benefit observed for either upadacitinib-treated group within the first week,” reported the principal investigator of SELECT-BEYOND, Dr. Mark Genovese, Co-Chief, Division of Immunology and Rheumatology, Stanford University, Stanford, California. The steep improvement in disease control on multiple measures is consistent with rapid onset of JAK1 selectivity (Fig. 1a and 1b). Patients discontinued biologics but remained on stable csDMARD during the study. For entry into SELECT-BEYOND, patients were permitted to have failed one or more biologics from the same or different drug classes. Of the 499 randomized, approximately 25% had failed two and another 25% had failed at least three. Despite this history, ACR20 was achieved at 12 weeks in 65% and 56% of those randomized to 15 mg and 30 mg, respectively, of upadacitinib. The figures for ACR50 were 34% and 36%, respectively. For ACR 70, the proportions were 12% and 23%. Response rates were similar among those with the most prior bDMARD failures compared to the least, according to Dr. Genovese. The findings were paralleled by SELECT-NEXT, which randomized 661 patients refractory to csDMARDs to the 15 or 30 mg dose of upadacitinib or placebo. This included the same rapid response. Although similar proportions of patients on 15 and 30 mg upadacitinib reached ACR20 by week 12 in SELECT-NEXT as SELECT-BEYOND, response rates were somewhat higher for ACR 50 (38% and 43%, respectively) and ACR 70 (21% and 27%, respectively).
Consistent Symptom Relief at 12 Weeks
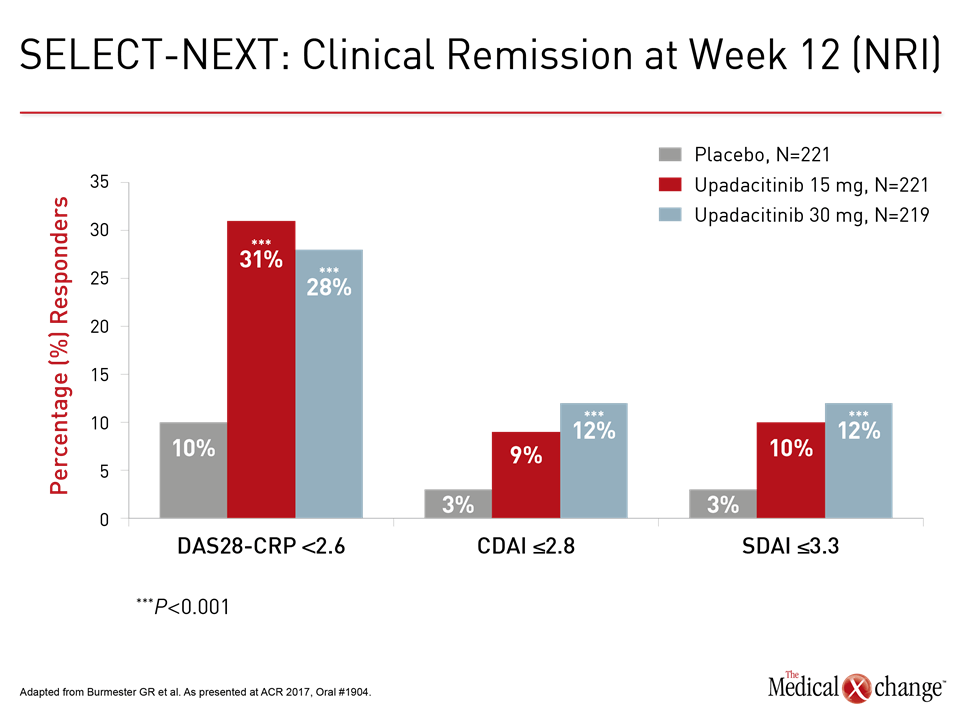
“The patients achieving a clinical remission at 12 weeks was about three times higher on upadacitinib than placebo whether measured with DAS-CRP, CDAI, or SDAI,” reported the principal investigator of this trial, Dr. Gerd R. Burmester, professor of rheumatology and clinical immunology, Charité Hospital, University of Berlin, Germany (Fig. 2).
“The patients achieving a clinical remission at 12 weeks was about three times higher on upadacitinib than placebo.”
In the first 12 weeks of SELECT-BEYOND, the total rate of adverse events (56.2% vs. 55.5%) and rate of discontinuation for adverse events (5.2% vs. 2.4%) were slightly higher in the placebo versus the 15 mg upadacitinib treatment arms. Rates of total adverse events (67.3%) and discontinuations (9.1%) were higher in the 30 mg upadacitinib arm. In SELECT-NEXT, the overall rate of discontinuation for adverse events was lower overall in the placebo and 15 mg upadacitinib arms (3.2% in both) and the 30 mg arm, which was 5.9%. Side effects of interest based on previous experience with JAK inhibitors were reassuring. Herpes zoster rates in SELECT-BEYOND were higher in the 30 mg upadacitinib group (2.4%) but the same in the placebo and 15 mg upadacitinib group (0.6%). In SELECT-NEXT, there was only one case each of herpes zoster in the placebo and 15 mg upadacitinib arms and two cases in the 30 mg arm. There were no cases of pulmonary embolism or deep venous thromboembolism (PE/DVT) in SELECT-NEXT. These were higher in the active treatment arms of SELECT-BEYOND, but Dr. Genovese reported that risk is difficult to interpret because of preexisting risk factors. There was no pattern for development of PE/DVT relative to upadacitinib initiation. Differences in hemoglobin or platelet levels over the course of treatment were not significant in either study.
Side Effects Relevant to JAK Selectivity
These specific side effects are of interest due to the potential of JAK selectivity to reduce risk of off-target effects. Through the JAK-STAT (Signal transducer and activator of transcription) pathway, JAK1 activates pro-inflammatory cytokines such as interleukin-6 (IL-6) and interferon. These participate in RA-related inflammation as well as in other inflammatory diseases, such as psoriasis. The other three isoforms are linked to other biologic functions, particularly hematopoiesis in the case of JAK2, lymphocyte function in the case of JAK3, and regulation of interferon, IL-12, and IL-23 in the case of TYK2, which is the fourth member of the JAK enzyme family. Relative to tofacitinib, which has inhibiting activity on JAK pathways other than JAK1, particularly JAK3, newer more specific JAK inhibitors in late stages of clinical development are being carefully monitored for a more targeted activity profile. Large-scale comparisons of drugs with different profiles of relative JAK inhibition are needed to prove this hypothesis, but the studies so far with next-generation agents suggest that they have at least comparable anti-inflammatory activity as the first-generation agent. This includes, baricitinib, for which the first phase 3 results were announced almost a year ago, and filgotinib, for which phase 3 trials are ongoing. Although baricitinib also inhibits JAK2 and is less specific than upadacitinib and filgotinib, it has already been approved for treatment of RA in Europe.
Baricitinib Results at 12, 24 Weeks
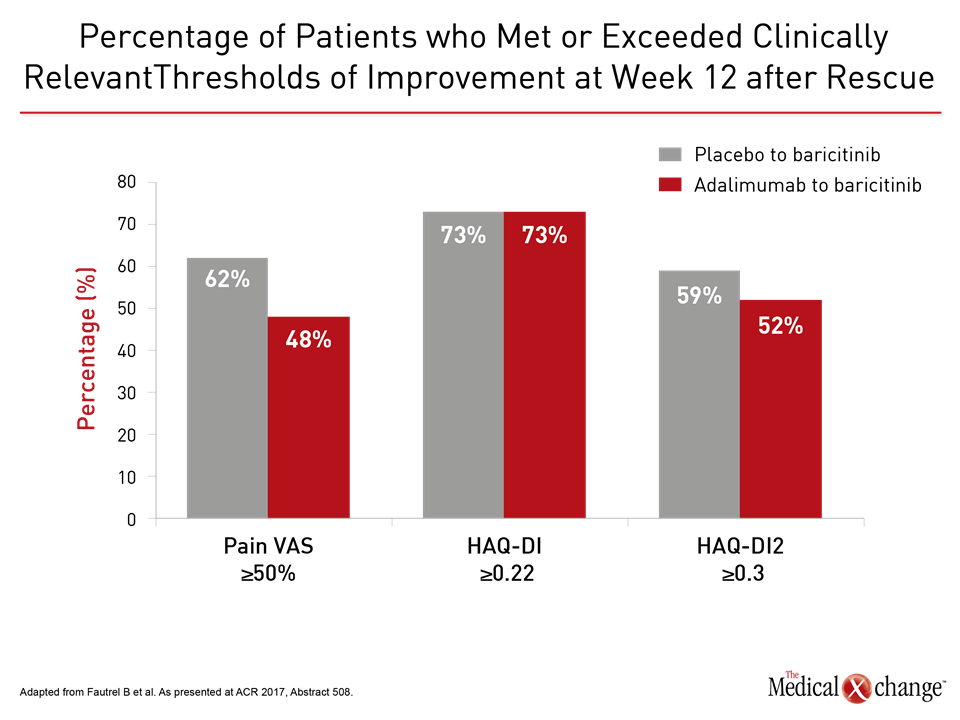
“The differences between baricitinib and adalimumab reached significance at 12 and 24 weeks for several clinical measures,” reported Dr. Peter Nash, Department of Rheumatology and Immunology, University of Queensland, Brisbane, Australia. This included the mean overall Clinical Disease Activity Index (CDAI) score at 12 weeks (P=0008) and 24 weeks (P=0.035) as well as those with a mean CDAI improvement ≥12 at week 12 (P<0.001) and week 24 (P<0.010). In RA-BEAM, 1355 patients with an inadequate response to methotrexate were randomized to 4 mg once daily oral baricitinib, placebo, or 40 mg adalimumab every two weeks. The data presented by Dr. Nash at the ACR meeting were among a series of secondary and post-hoc analyses highlighting the activity of this drug. In another study, presented by Dr. Bruno Fautrel, Department of Rheumatology and Immunology, Pierre and Marie Curie University, Paris, France, benefit was evaluated in those initially randomized to placebo or adalimumab that were rescued with baricitinib. Although only 12% of adalimumab patients versus 26% of placebo patients underwent rescue, the improvement after switch was about the same for those treated with adalimumab as those treated with placebo for several outcomes (Fig. 3). “Upon failure on adalimumab, rescue with baricitinib results in early control and improvement in symptoms relevant to patients,” Dr. Fautrel contended.
Baricitinib Safety Meta-Analysis Reassuring
In a safety meta-analysis from five baricitinib trials with 2006 patients, safety was encouraging. Relative to placebo, baricitinib was associated a modest odds ratio (OR) increase in overall risk of any adverse events (OR 1.35; P=0.05), but no significant increase in serious adverse events (OR 1.12; P=0.58). Consistent with the potential for selective JAK inhibitors to reduce off-target effects, “baricitinib did not appear to generate any significant safety concerns during the first six months of treatment,” concluded the first author of the meta-analysis, Dr. Sumit Kunwar, Department of Rheumatology, MedStar Washington Hospital Center, Washington, DC. Encouraging safety data were also presented at the 2017 ACR meeting on filgotinib. Based on long-term followup from DARWIN3, an open-label extension that enrolled patients from the phase2b DARWIN1 and DARWIN2 trials, there were no changes observed in an array of laboratory analyses over a median 917 days on study drug. Five hundred and twenty of the 603 patients who entered DARWIN3 completed at least 84 weeks of follow-up.
Filgotinib Safety on Off-Target Effects
In this analysis, the rate of adverse events was stratified by dose of filgotinib (100 mg twice daily, 100 mg once daily, or 200 mg once daily) with or without methotrexate. Although there was a dose-response effect so that serious adverse events and grade 2 or higher laboratory abnormalities were more common with the higher doses, grade 3 or higher adverse events were rare. These included those potentially related to non-specific JAK inhibition such as change in hemoglobin, lymphocyte activity, platelet count, and kidney function.
“Over time, the safety profile [of filgotinib] remained favorable.”
“Over time, the safety profile remained favorable,” reported Dr. Genovese, who also presented these filgotinib results. In the context of durable efficacy that was also seen in this long-term follow-up, Dr. Genovese indicated that the findings encourage the on-going phase 3 development program.
Conclusion
A new generation of JAK inhibitors with greater selectivity on JAK1, the isoform considered most relevant to RA-associated inflammation, have reached late stages of clinical development. The relative selectivity of these agents may be relevant to both efficacy and safety. Although comparative trials are needed to pursue this hypothesis, the efficacy and safety of these agents in clinical trials has been encouraging. This includes two newly completed phase 3 trials with upadacitinib, a selective JAK1 inhibitor, and baricitinib, a JAK1/JAK2 inhibitor that previously completed phase 3 testing. These drugs promise to expand therapeutic options for RA and possibly other inflammatory conditions.