Respirology
American Thoracic Society (ATS) 2018 International Congress
Phase 3 Trials of As-needed Anti-inflammatory Regimen Tested in Mild Asthma
San Diego – In patients with mild asthma, an as-needed, single-inhaler combination of a corticosteroid and a long-acting beta agonist (LABA) is effective for maintaining disease control and reducing glucocorticoid exposure, according to two newly-completed phase 3 trials. Presented at the 2018 annual meeting of the American Thoracic Society (ATS) and published simultaneously in the New England Journal of Medicine, as-needed single inhaler combination was non-inferior to twice-daily maintenance inhaled corticosteroids (ICS) for preventing severe exacerbations in both studies. Both studies also associated as-needed therapy with at least a 75% reduction in glucocorticoid exposure relative to maintenance ICS. Symptoms were better controlled on the as-needed single inhaler combination of ICS/LABA relative to as-needed short-acting beta agonists (SABA) but somewhat less well controlled relative to maintenance ICS.
SYGMA Trials Deliver the Same Message
Control of airway inflammation with ICS therapy is recommended in most adults and adolescents, including those with mild disease. Compliance is notoriously poor, in that many patients rely on rescue SABA when symptoms develop. However, SABA therapy does not reduce the inflammatory process that drives severe exacerbations, which occur even in patients with mild asthma and that underlies the recommendation for maintenance ICS. New data suggest the solution to this problem may be a single inhaler containing both a glucocorticoid and a LABA in an as-needed fashion.
“The question is whether an ICS and a fast-acting LABA taken as-needed could address the underuse of ICS in patients with mild asthma.”
“The question we were addressing was whether a single inhaler combination of an ICS and a fast-acting LABA taken only as-needed for symptom relief could address the underuse of ICS and improve asthma control in patients with mild asthma,” reported Dr. Paul M. O’Byrne, Dean, Faculty of Health Sciences, McMaster University, Hamilton, Ontario.
Dr. O’Byrne was principal investigator of the SYGMA 1 trial (O’Byrne PM et al. N Engl J Med 2018;378:1865-1876), which was one of the two related trials with similar goals but slightly different designs. In this trial, the primary endpoint was control of asthma symptoms. In a second trial with a different randomization, called SYGMA 2, the primary endpoint was rate of severe exacerbations (Bateman ED et al. N Engl J Med 2018;378:1877-1887). Both trials support the conclusion that as-needed treatment with a single inhaler containing an ICS and a LABA may be a viable alternative for maintenance ICS, although Dr. O’Byrne cautioned that it is still experimental.
“This approach has not yet been approved by Health Canada so cannot be recommended for use as yet,” he responded when asked whether results are relevant to current practice.
More than 8,000 Patients Randomized
In the double-blind SYGMA 1 trial, 3849 patients with mild asthma 12 years of age or older were randomized to one of three arms; maintenance placebo plus the SABA terbutaline used as needed, maintenance placebo plus a single inhaler combination of the ICS budesonide and the LABA formoterol used as needed, or maintenance twice-daily budesonide plus terbutaline used as needed. All assigned maintenance regimens were to be administered twice daily. Patients were also instructed to take two daily doses when using their as-needed inhaler. The primary endpoint of SYGMA 1 was percentage of weeks with asthma control over the 52-week study period. Relative protection against severe exacerbations was a secondary objective.
In SYGMA 2, 4215 patients were randomized to one of two arms; maintenance placebo plus ICS/LABA as needed or maintenance twice-daily budesonide plus terbutaline used as needed. The specific drugs in the two arms were the same as those used in SYGMA 1. In both trials, regardless of arm, the doses of the active agents were 200 μg for budesonide, 6 μg for formoterol, and 0.5 mg for terbutaline. The primary endpoint of SYGMA 2, which was also double blind, was annualized rate of severe asthma exacerbations.
As-Needed ICS Superior to SABA
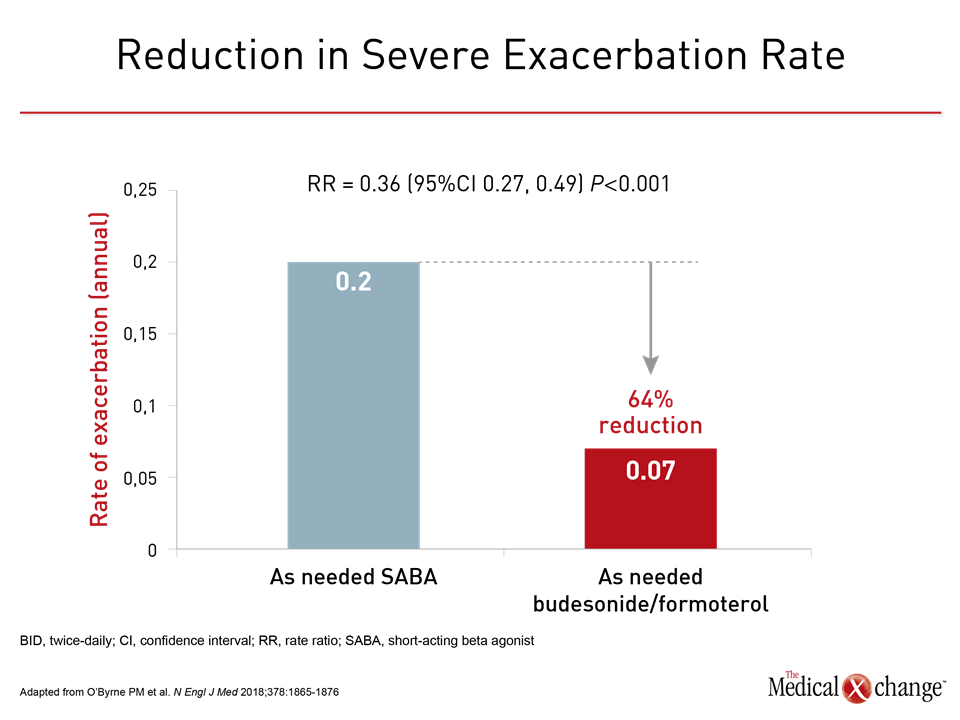
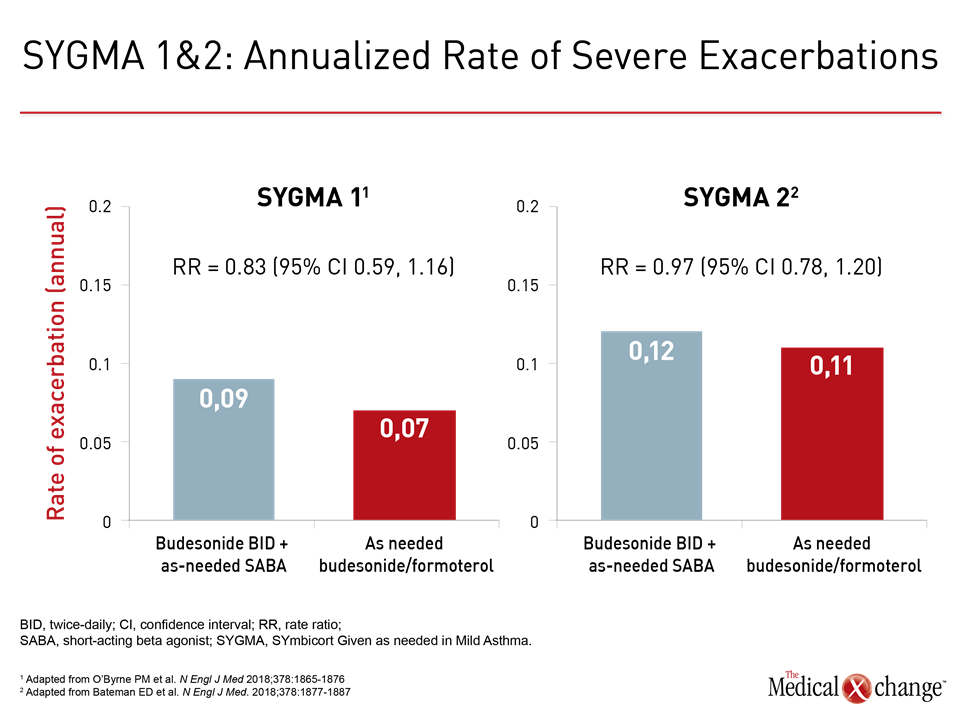
Both studies achieved their primary endpoint. In SYGMA 1, as-needed budesonide/formoterol achieved a higher percentage of weeks with well-controlled asthma (34.4% vs. 31.1%; P=0.046) than as-needed terbutaline. It was also associated with a 64% relative risk (RR) reduction in the annualized rate of severe exacerbations (0.07 vs. 0.2; RR 0.36, P<0.001) relative to the SABA (Fig. 1).
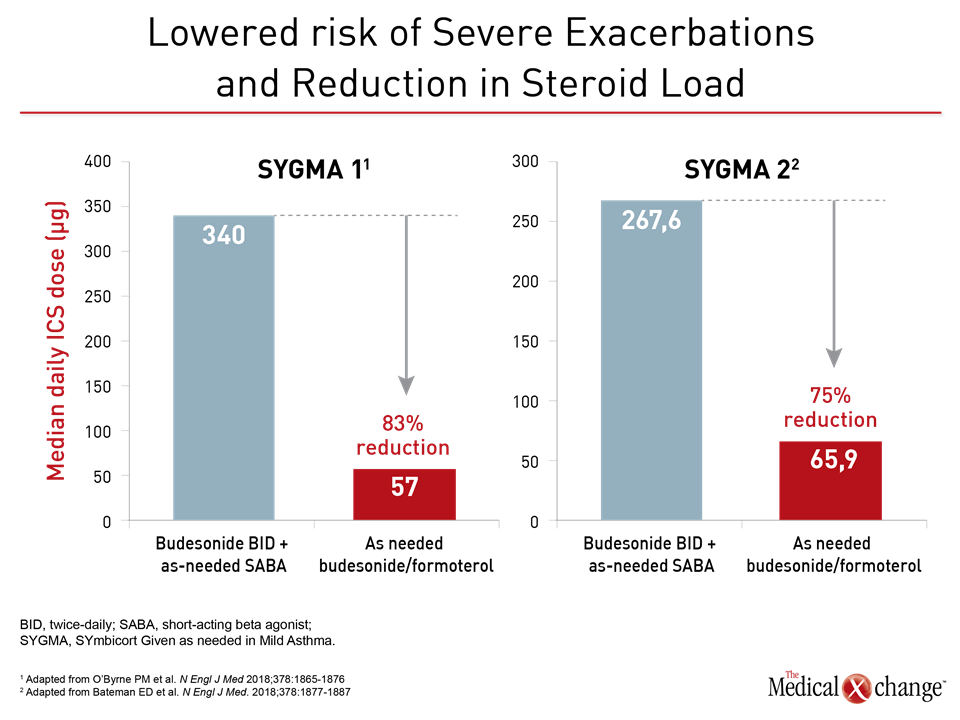
When compared to budesonide maintenance, the as-needed combination performed less well for proportion of weeks with well-controlled asthma (34.4% vs. 44.4%). However, the annualized rate of exacerbations was numerically lower in the as-needed budesonide/formoterol arm and these outcomes were achieved with an 83% lower glucocorticoid exposure relative to budesonide maintenance. Moreover, Dr. O’Byrne characterized the differences in other outcomes, such as change from baseline in the 5-item Asthma Control Questionnaire (ACQ-5) (mean difference 0.15) and change from baseline in FEV1 (mean difference 54.3 mL) as “small.”
Annualized Exacerbation Rates Similar
In SYGMA 2, which evaluated annualized exacerbation rate as the primary endpoint, the as-needed budesonide/formoterol performed as well as maintenance budesonide (0.11 vs. 0.12), meeting the prespecified definition of noninferiority (Fig. 2a and 2b). In a detailed analysis, there was no difference between arms in the time to first severe exacerbation, the rate of exacerbations requiring hospitalization or emergency room visits, the proportion of patients with at least one exacerbation, or the proportion of patients requiring systemic corticosteroids for at least 3 days to manage a severe exacerbation.
“The rate of severe exacerbations in the budesonide/formoterol group was achieved with less than one quarter of the total exposure to the glucocorticoid.”
In addition, “the rate of severe exacerbations in the budesonide/formoterol group was achieved with less than one quarter of the total exposure to the glucocorticoid,” reported Dr. Eric D. Bateman, Division of Pulmonology, University of Cape Town, Cape Town, South Africa (Fig. 3a and 3b). Lead investigator of SYGMA 2, Dr. Bateman also pointed out that the protection from severe exacerbations with as-needed budesonide/formoterol circumvents the need for twice-daily ICS maintenance.
In SYGMA 1, adherence, which was monitored electronically and with twice-daily reminders, was 79% across all groups. It was about 15% lower in SYGMA 2, but even this rate substantially exceeds that observed in the real-world setting. According to data cited by Dr. Bateman, typical rates of adherence among patients with mild asthma is near 35%. While the reasons for poor adherence to maintenance therapy may include the failure of patients with mild asthma to recognize the risk of severe exacerbations or fear about glucocorticoid exposure, it is likely that the far lower adherence to maintenance ICS therapy expected outside of a clinical trial would confer a much greater advantage on the as-needed regimen.
Real-World Results May Differ
Although he explained that the double-blind, double-dummy design was essential for testing the relative efficacy of as-needed versus maintenance therapy in mild asthma, Dr. O’Byrne made the same point. In the discussion section of the published study, he also speculated that adherence to maintenance budesonide would be substantially lower in routine practice and might have changed the findings regarding relative symptom control. It is notable that the study did demonstrate superiority for as-needed budesonide/formoterol over as-needed SABA, which is the comparison relevant to patients who are not adherent to maintenance anti-inflammatory therapy.
Baseline demographics in these two trials were similar and representative of patients with mild asthma. Only about 20% of patients had a severe asthma exacerbation in the previous 12 months. The average time since diagnosis of asthma was approximately 6.5 years. The pre-bronchodilator FEV1 was nearly 85% of the predicted value. About 60% of the study population was female. The average age was near 40 years.
All of the regimens used in both trials were well tolerated. According to Dr. O’Byrne, speaking specifically about SYGMA 1, “There were no differences in the adverse event profile between treatments except that there were more adverse events leading to discontinuation in the as-needed terbutaline arm [2.9%] when compared to the budesonide maintenance arm [1.2%] or the as-needed budesonide/formoterol arm [0.8%].”
Smaller Study with Similar Results
Pursuing the same theoretical advantages, others are exploring this as-needed approach. For example, a randomized, single-blind study presented here also associated as-needed ICS with adequate symptom control and a reduced ICS exposure. This study was conducted in a resource-limited setting and confined enrollment to a pediatric population. In the study, 206 children with mild asthma were randomized to daily therapy with fixed-dose ICS beclomethasone or as-needed therapy in which patients took ICS beclomethasone whenever the SABA albuterol used for rescue. The average age of the children in this study, most of who came from a low-income household, was 10.3 years.
There were no differences in asthma control over the 6-month course of the study as assessed with change from baseline in the Asthma Control Test (ACT), in rate of exacerbations, or in change from baseline in quality of life, according to Dr. Kaharu Sumino, Associate Professor of Medicine, Washington University School of Medicine, St. Louis, MO. However, patients randomized to as-needed ICS had a significantly lower ICS consumption (P<0.0001).
“As-needed ICS results in lower ICS exposure [than daily ICS use] and a sense of self management.”
The use of as-needed ICS “results in lower ICS exposure and a sense of self-management,” according to Dr. Sumino. Like the authors of the SYGMA studies, she suggested that as-needed therapy might be a practical way to increase the proportion of asthma patients who receive guideline-recommended anti-inflammatory therapy to address the underlying pathophysiology of asthma.
Conclusion
Mild asthma is defined by a low symptom burden but not by a low risk of significant and even life-threatening exacerbations. Most guidelines advocate regular use of inhaled corticosteroids to prevent airway inflammation due to exacerbation risk, but adherence is poor. Despite the fact that up to 40% of exacerbations leading to emergency room visits occur in patients with mild disease, patients commonly manage symptoms with rescue SABA agents alone, foregoing the anti-inflammatory component important to exacerbation prevention. The large SYGMA trials provide evidence that as-needed budesonide/formoterol might be a viable alternative to daily anti-inflammatory therapy. For management of symptoms, it appears to be far superior to rescue SABA while offering a degree of protection against exacerbations that is commensurate with daily ICS therapy.