Rheumatology
European League Against Rheumatism (EULAR) 2018
Psoriatic Arthritis Remains Controlled on Anti-IL-17 Agents out to Three Years
Amsterdam – The role played by IL-17 signaling in the inflammatory cascade of psoriatic arthritis (PsA) appears to be even more fundamental to disease pathogenesis than initially appreciated. New data presented at the 2018 EULAR Congress associate inhibition of IL-17 with indefinite control of PsA and inhibition of radiographic progression. Data from one of the anti-IL-17 phase 3 trials program is now out to three years with approximately 50% of patients demonstrating suppression of joint disease activity at a level of ACR50. Enthesitis at three years remained completely resolved in nearly half of patients.
Approved Treatments Show Long-term Efficacy
“Like the two-year results we published last year, the three-year efficacy and safety data show similar sustained improvement,” reported Dr. Peter Nash, University of Queensland, Brisbane, Australia. He was referring specifically to data with secukinumab, which, along with ixekizumab, is one of two anti-IL-17 therapies approved for PsA in Canada and elsewhere. High rates of efficacy in initial clinical trials, many of which enrolled patients with inadequate disease control on tissue necrosis factor inhibitors (TNFi), can now be placed in the context of long-term benefits.
FUTURE 2: Three-year Data Demonstrate Sustained Benefits
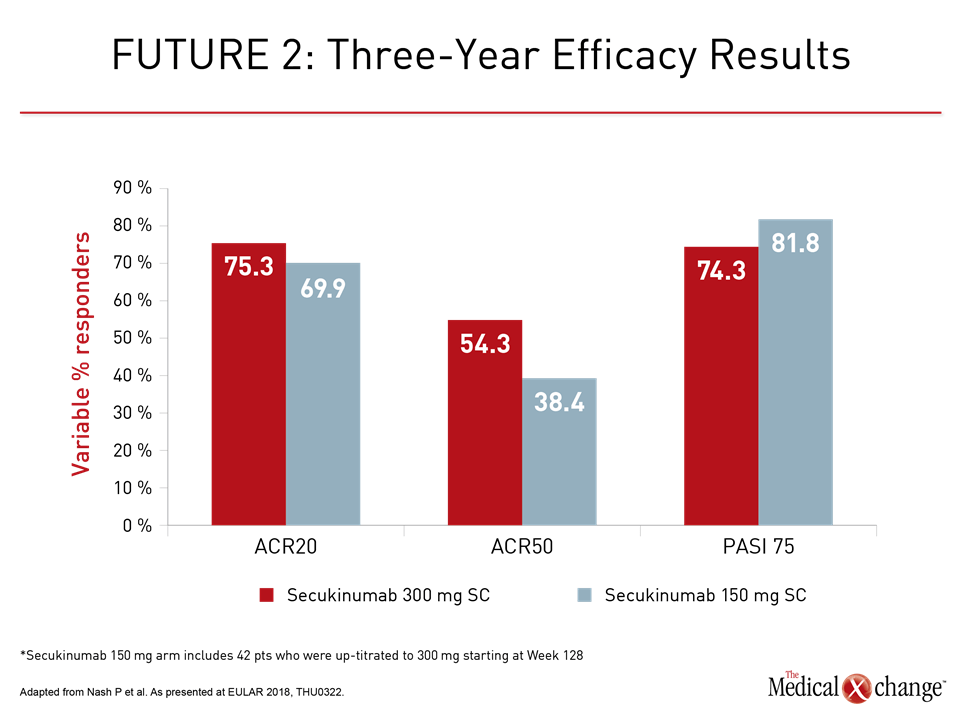
The consistency of sustained benefit was the important message of three-year data from the FUTURE 2 trial with secukinumab. Of the 200 patients initially randomized to 300 mg or 150 mg doses of every-four-week subcutaneous (SC) secukinumab, approximately 72% remained on therapy. The proportion with an ACR20 response was approximately 70% at last follow-up in both arms. The 300 mg SC dose was associated with a higher ACR50 response (54.3% vs. 38.4%) at three years, but PASI 75 control of skin involvement was similar (74.3% vs. 81.8%, respectively) (Fig. 1).
SPIRIT-P2 Trial
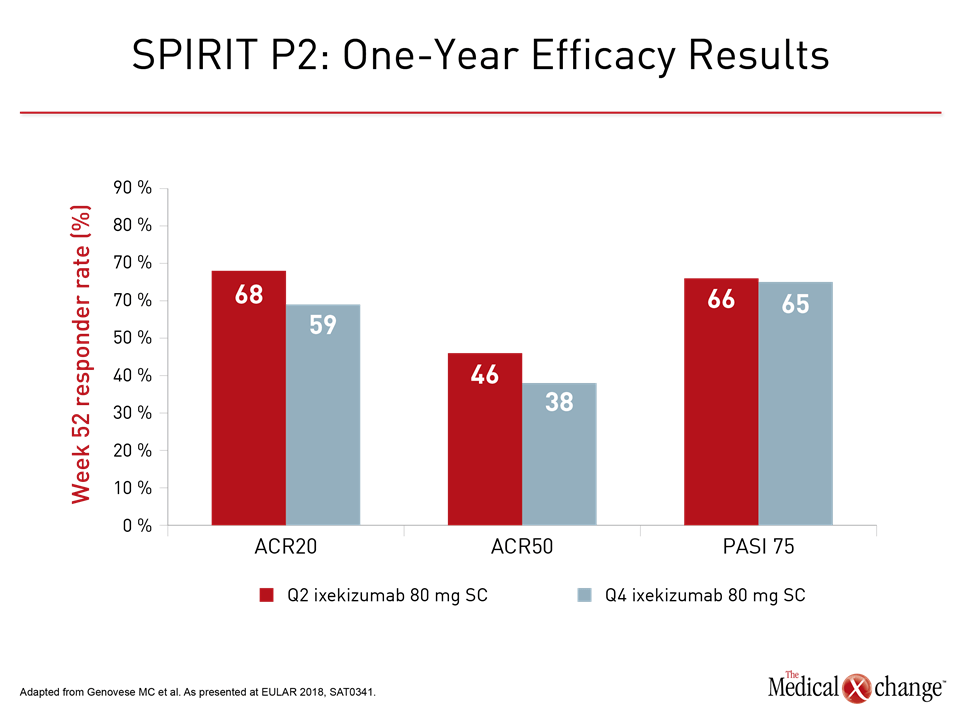
Although follow-up was shorter, the phase 3 SPIRIT-P2 trial with ixekizumab also showed long-term benefit. The 42-week analysis presented at the 2018 EULAR Congress included data from patients who crossed over from placebo by week 24. At 52 weeks, the ACR20 responses were approximately 60% with 80 mg SC ixekizumab administered at either two or four weeks and regardless of having been started or switched to active therapy. The proportions of patients achieving PASI 75 response were more variable, ranging from 48% to 76% without a clear dose relationship.
Like Dr. Nash, the first author of SPIRIT-P2, Dr. Mark C. Genovese, Stanford University, California emphasized the sustained benefit. No new safety concerns over 52 weeks emerged (Fig. 2).
FUTURE 5 Data
Not least relevant to the efficacy of anti-IL-17 therapy, the data from FUTURE 5 also presented here associated anti-IL-17 therapy with protection against structural damage. When radiographs taken at baseline, week 16, and week 24 were evaluated by blinded readers, there were lower rates of progression relative to placebo for all doses of secukinumab, according to Dr. Désirée van der Heijde, Leiden University Medical Center, The Netherlands. “The low rates of progression on active therapy were observed whether or not patients had been previously treated with TNFi,” Dr. van der Heijde reported.
Conclusion
Early disease control provided the basis for regulatory approval of anti-IL-17 therapies, but these long-term data also demonstrate a modification of the natural history of PsA.