Obesity
ObesityWeek 2018
In Obesity, Brain Circuitry Influences Cravings and Dysregulated Eating
Nashville – New techniques show differences in brain network activity to cravings, food addiction, and stress response for patients with overweight or obesity. Strategies that target brain reward circuitry to mitigate acute appetite response are an effective component of a comprehensive weight management strategy.
For some patients who have overweight or obesity, unwanted eating behaviors may be mediated by reward circuitry in the brain, a neural pathway that differs from appetite and is implicated in other addictive behaviors. Brain imaging and biochemical assays show a link between activation of reward pathways and neurohormonal changes associated with cravings.
Pharmacologic strategies used in other addictive behaviors represent a unique approach to the pressing problem of overweight and obesity. The combination of bupropion, an atypical antidepressant, and the opioid antagonist naltrexone can contribute to significant weight loss in patients who use the medication combination as an adjunct to diet and weight loss. The combination acts, in part, by damping the reward circuitry that mediates craving, which can also be thought of as an acute appetite response. Recent work presented at this year’s ObesityWeek shows naltrexone/bupropion to have a safe pharmacokinetic profile in the adolescent population, no concerning blood pressure (BP) changes, and delivers new insights into renal safety to inform prescribing for patients who had obesity and concomitant renal impairment.
Acute Appetite Control is Important
A recent systematic review presented here showed that interventions to help achieve acute, as well as chronic, control of appetite are beneficial in efforts to manage body weight.
All four of the studies identified in the systematic review supported an affirmative answer to the question, “Are acute effects on appetite linked to beneficial effects on body weight management?” The studies variously measured acute appetite control by measuring energy intake, or by measures of subjective appetite, and all found significant beneficial effects for achieving acute control of appetite, noted Dr. Thea Toft Hansen, Department of Nutrition, Exercise and Sports, University of Copenhagen, and coauthors. “New strategies could lead to expansion of the ‘toolbox’ needed to help people manage body weight in order to maintain health and wellbeing throughout life,” she reported.
“New strategies could lead to expansion of the ‘toolbox’ needed to help people manage body weight to maintain health and wellbeing throughout life.”
Reward Circuitry Implicated in Obesity
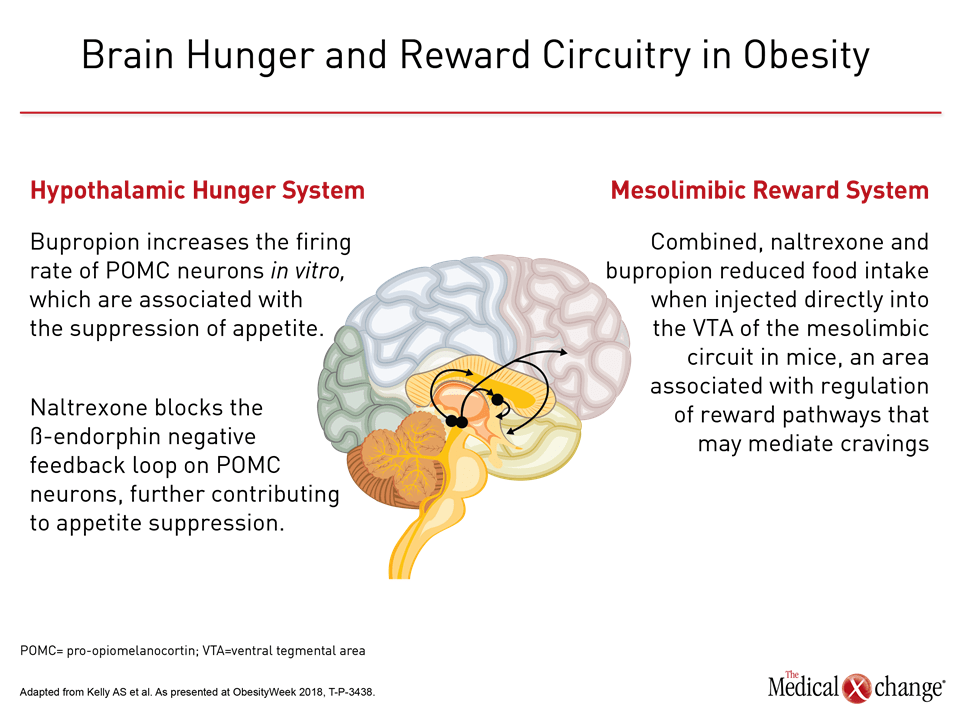
Therapeutic options that target the brain’s hunger and reward systems include the combination of bupropion and naltrexone. In a poster presentation that addressed the single-dose pharmacokinetics of this combination in adolescents with obesity, Dr. Aaron Kelly, Associate Professor of Pediatrics and Co-Director of the Center for Pediatric Obesity Medicine, University of Minnesota noted that the hunger system is thought to be driven primarily by the hypothalamus.
In the hypothalamic hunger system, beta endorphins can exert negative feedback on pro-opiomelanocortin (POMC) neurons. Downregulation of this neuronal activity can negate the usual appetite-suppressing function of POMC neurons. “Bupropion increases the firing rate of POMC neurons in vitro,” reported Dr. Kelly. “Naltrexone blocks the beta-endorphin negative feedback loop on POMC neurons, further contributing to appetite suppression” (Figure 1).
The mesolimbic reward system is also implicated in obesity, and naltrexone and bupropion used in conjunction may mitigate cravings through their action on the reward system. Dr. Kelly cited work showing that “combined, naltrexone and bupropion reduced food intake when injected directly into the [ventral tegmental area] of the mesolimbic circuit in mice, an area associated with regulation of reward pathways that may mediate cravings.”
Adolescent Pharmacokinetics Show Exposure Similar to Adults
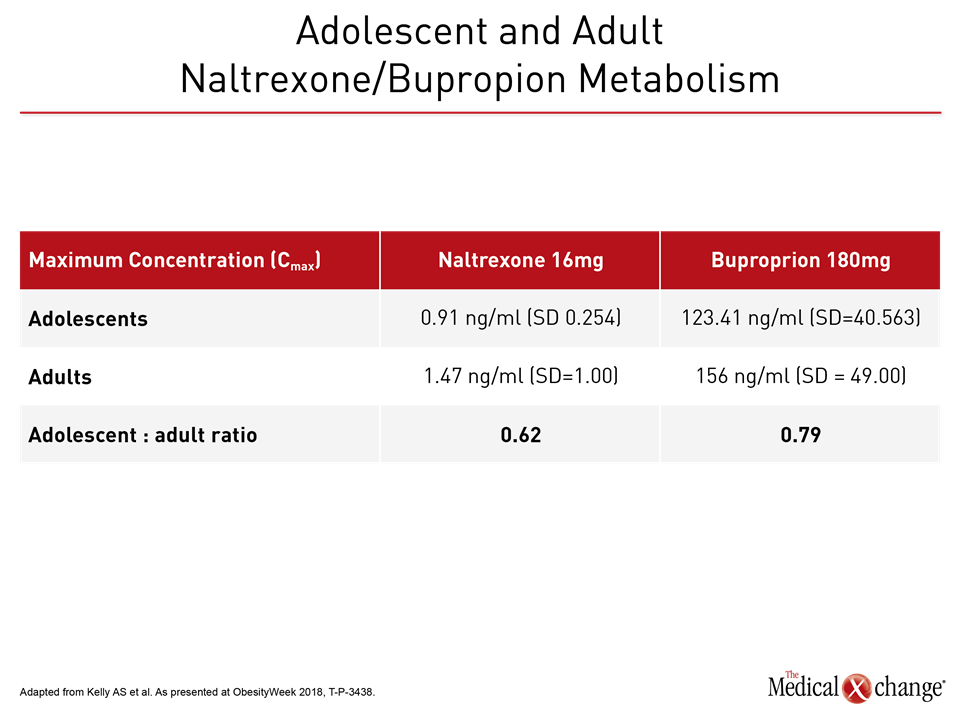
The combination of naltrexone and bupropion is approved worldwide for chronic weight management, when used as an adjunct to diet and physical activity by adults who are overweight or have obesity, and have at least one weight-related comorbidity. In adolescents, Dr. Kelly found that single-dose pharmacokinetics (PK) of naltrexone/bupropion showed exposure similar to, or slightly lower than, that seen in adults.
With naltrexone, the maximum concentration (Cmax) of naltrexone dosed at 16mg for adolescents was 0.91 ng/ml (standard deviation [SD], 0.254). In adults, the Cmax was 1.47 ng/ml (SD=1.00) for an adolescent: adult ratio of 0.62. For the bupropion moiety of the combination medication, the adolescent Cmax for a 180 mg dose was 123.41 ng/ml (SD=40.563), while the adult Cmax for the same dose was 156 (SD=49.00), yielding an adolescent: adult ratio of 0.79 for the maximum serum levels of bupropion. Similar ratios were seen for active metabolites of the two medications (Table 1).
Cardiometabolic Comorbidities
Many patients with obesity or overweight have cardiometabolic comorbidities, including hypertension and type 2 diabetes. In patients with overweight or obesity who participated in pivotal clinical trials, a small increase in BP was seen during the initial treatment phase with naltrexone/bupropion, an effect thought to be due to bupropion. By the end of the study period, however, BP in participants treated with naltrexone/bupropion returned to pre-study levels, or was lower than at initiation.
In a new examination of pooled pivotal clinical trial data for naltrexone/bupropion, investigators further elucidated the relationship between weight loss and BP. They found that while this combination “is associated with small increases in mean BP relative to [placebo] for a given amount of weight loss, the directional relationship between weight loss and BP reduction is maintained, and on average, BP is reduced below baseline with successful weight loss,” reported Dr. Lisette Acevedo, Director, Clinical Science, Nalproprion Pharmaceuticals, La Jolla, California.
For the 53% of naltrexone/bupropion patients who had at least a 5% weight loss at one year, mean systolic and diastolic BP were each reduced relative to baseline.
Both diabetes and hypertension can, over the long term, lead to renal impairment. Phase 1 study data presented here investigated how patients with renal impairment fared when given naltrexone/bupropion, comparing participants with mild, moderate, and severe renal impairment with those who had normal renal function.
Dr. Acevedo and colleagues stratified participants according to renal function. The investigators then matched participants with normal renal function to those with renal impairment classified as mild, moderate, or severe. Participants with end-stage renal disease were excluded.
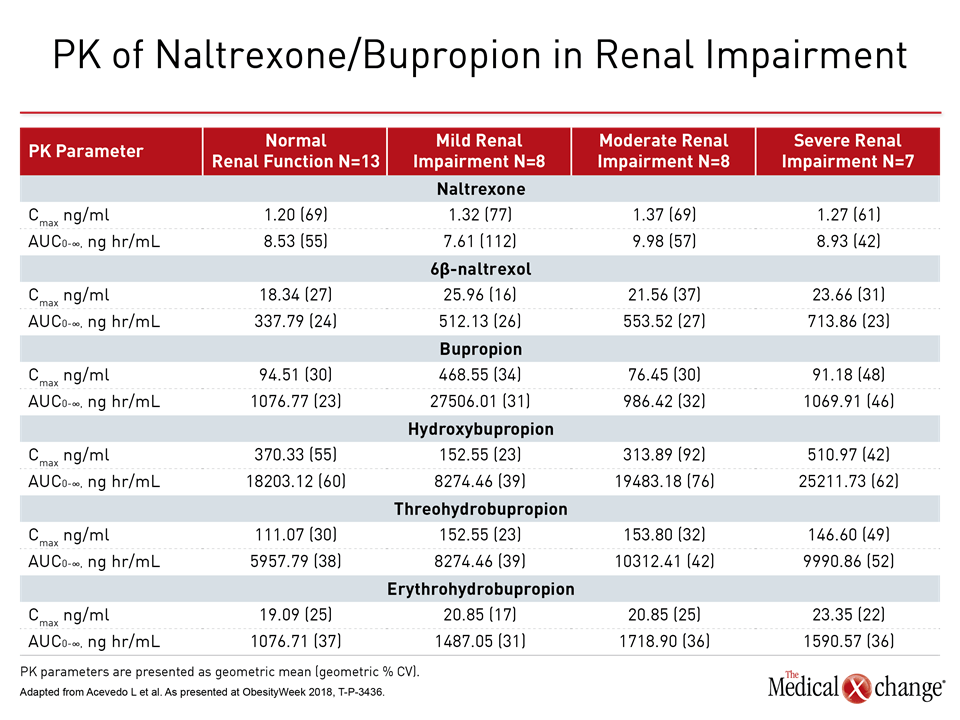
Participants received a single dose of extended-release naltrexone 32 mg/extended-release bupropion 360 mg, and PK were followed via blood and urine samples that tracked levels of bupropion, naltrexone, and active metabolites of each medication from study day 1 through to 168 hours after the single dose.
“Renal impairment had no significant effect on PK parameters for naltrexone, bupropion, and hydroxybupropion,” noted Dr. Acevedo. Total exposure was increased for some active metabolites, and serum half-life was increased for just two metabolites, 6β-naltrexol and erythrohydrobupropion.
“Renal impairment had no significant effect on PK parameters for naltrexone, bupropion, and hydroxybupropion.”
However, the investigators used a weighted analysis to account for the relative pharmacologic activity and potency of both parent compounds, as well as their metabolites.
“As these active metabolites are less potent than the parent molecules (naltrexone or bupropion), increases in their concentrations resulted in less than two-fold increase in overall pharmacological activity of [naltrexone/bupropion] in participants with moderate or severe renal impairment and a smaller increase in participants with mild renal impairment” (Table 2).
Food Cravings and the Brain-gut Link
New research examines how brain networks differ between those with normal and high body mass index (BMI), finding that reward and salience regions of the brain are activated in patients with high BMIs when exposed to stress-related metabolites.
Dr. Arpana Gupta, Assistant Professor, Center for Neurobiology of Stress and Resilience, Division of Digestive Diseases, University of California Los Angeles, called for greater attention to “bidirectional signaling between the brain and the gut microbiome…, which is mediated via neural, metabolomic, endocrine, and immune-related signaling mechanisms.”
To look at how brain function impacts – and is impacted by – addictive food behaviors and other neurobehavioral factors, Dr. Gupta and her collaborators examined 63 healthy individuals, 32 with BMIs of 18.50 – 25.99 kg/m2, and 31 with BMIs of 25 kg/m2 or higher. The normal BMI group consisted of 20 females and 12 males, and the high BMI group of 14 females and 17 males.
Participants had resting state functional magnetic resonance imaging (fMRI) scans of their brains. A variety of brain networks were examined; these included the reward network as well as the emotional regulation, salience, and somatosensory networks.
These networks are made up of neuronal connections between anatomically distant parts of the brain that are activated in various emotional and physiological states. For example, when an individual is presented with food images that provoke cravings, all four networks will see changes, with reward, salience, and somatosensory network activation, and emotional regulation network downregulation.
Additionally, stool samples were collected and examined for fecal metabolites. Dr. Gupta and her colleagues, in previous work, had seen differences in these metabolites among individuals who were experiencing stress or pro-inflammatory states. The investigators hypothesize a brain-gut-microbiome axis that involves bidirectional communication, setting up negative feedback loops where a higher level of stress can cause a brain state where cravings are harder to ignore, and overeating is more likely.
When, for example, stress or inflammation push degradation of the amino acid tryptophan into the kynurenine pathway, “neuroreactive metabolites” are produced that have “been proposed as important modulators of brain-gut communication,” reported Dr. Gupta.
Similarly, life circumstances can “shape the brain to cause changes…in both the gut microbiome and in eating behaviors,” investigators noted.
In addition to the fMRI scans and fecal metabolite analysis, participants completed the Yale Food Addiction Scale to assess cravings and food reward sensitivity. The scale prompted respondents to think of foods they might eat to excess, such as salty, fatty, and sugary foods, starchy food, and snack food. With this in mind, respondents were asked about the frequency of addictive food behaviors, by endorsing such statements as “I find myself constantly eating certain foods throughout the day,” and “I have found that I have elevated desire for or urges to consume certain foods when I cut down or stop eating them.”
Some of Dr. Gupta’s work focuses on early life adversity, so a scale assessing early traumatic experiences was also administered along with the Physical Health Questionnaire, to assess somatic symptoms.
Data analysis used a graph analytic approach, where connections were drawn among fMRI results, fecal metabolites, and questionnaire responses. This technique uses a “big data” approach to allow investigators to detect the number and strength of associations within a large number of variables.
Here, Dr. Gupta and her colleagues found clear differences between individuals with normal BMI and those who had overweight or obesity. For the latter group, there were many associations between high scores on the Yale Food Addiction Scale and somatosensory, salience, and emotional regulation brain network connectivity. The stress-related metabolite kynurenine also showed strong association with emotional regulation, salience, and somatosensory connectivity. All these associations were lacking in the normal-BMI participants.
There were also differences between male and female high-BMI participants, with all gut metabolites showing positive associations with the brain’s extended reward regions for female patients. Additionally, there were many connections between higher scores on the Yale Food Addiction Scale and all brain networks that were examined by fMRI in females. Associations were much sparser for the male respondents.
“A systems biology-based comprehensive understanding of the underlying pathophysiology of human obesity is required.”
Insights from Dr. Gupta’s findings may help clinicians identify individuals with overweight or obesity who might most benefit from pharmacologic treatment to target the brain’s reward system, she said.
Dr. Gupta and her colleagues are continuing to tease out the many associations among individual life experiences, perceptions of food and dysregulated eating, and neurohormonal factors, noting that “a systems biology-based comprehensive understanding of the underlying pathophysiology of human obesity is required,” wrote Dr. Gupta and her collaborators.
Conclusion
Acute control of appetite is important, and the evolving field of systems biology affirms that brain reward networks show differing activation in patients with obesity and those with dysregulated or addictive eating behaviors. Ongoing research that further elucidates the bidirectional nature of the brain-gut connection can guide clinicians in targeted pharmacotherapy for patients who struggle with overweight, obesity, and cravings.
General Practitioner’s Perspective
David A. Macklin MD CCFP
Director, Medcan Weight Management Program
Director Weight Management Mount Sinai Hospital BMI Clinic
Lecturer, University of Toronto Faculty of Medicine
Q. How does your practice support patients as they find their best weight?
A. I am a family practice physician; since 2005, I’ve led a behavioral weight loss management clinic. We take in between 400 and 500 new patients annually; I will see 15 to 18 patients daily. Our approach constitutes an intensive lifestyle intervention to support patients as they address the multiple genetic vulnerabilities and environmental cues that challenge Canadians today as they try to achieve their best weight.
A comprehensive approach that’s grounded in neuroscience is critical. Twenty thousand years ago, it was entirely adaptive and appropriate to have a strong biological drive to seek and consume high-energy food. Today, though, tasty food is everywhere – it’s cheap, easily available, and widely advertised.
Another shift that’s occurred in Canadian culture is that we no longer restrict our eating to three meals daily. Snacking and grazing are ubiquitous. And, of course, portion sizes at fast food establishments and in restaurants have gotten bigger and bigger. Finally, there’s a practice I call “health-washing,” when those who produce and market food products make misleading claims about the health benefits of these products.
Q. Talk a little more about the neurobiology of eating, overweight, and obesity.
A. First, the brain’s appetite system really comes from the midbrain. We hear terms like “craving,” “wanting,” “urge,” “incentive” – and these all are really getting at the idea that this urge to eat is subconscious. This is very clear from a review of the evidence in the medical literature.
For example, functional magnetic resonance imaging studies show that some individuals are more cue-sensitive when shown pictures of appetizing food. They will feel a stronger motivational drive to eat. And this is about reward learning: there’s a cue that prompts a craving or wanting, and then the individuals receives a reward – in this case, tasty food. The reward circuitry is amplified, and a deep groove is laid down. We are really talking about a Pavlovian response that is ultimately not under conscious control.
Understanding this is critical to removing the stigma from overweight and obesity. These are complex chronic conditions resulting from an interplay of genetics and the modern environment. We should not expect a chronic condition to improve or resolve without treatment.
Q. How do you use medication and behavioral interventions in your practice to address cravings and wants?
A. About 50% of patients in our practice are taking a weight loss medication. We now have available a new generation of medications that are not stimulants, but that directly get at the brain circuitry that regulates the reward system.
For some patients, these medications are a game changer. Through their direct action on brain areas involved in appetite, reward, pleasure, they can curb cravings and the urge to eat. Patients describe a huge sense of relief when this happens; they begin to feel some measure of control and agency.
These medications are used along with behavioral interventions, which provide a top-down approach. Using cognitive behavioral therapy, our staff help patients restructure their thinking in relation to food choices. Patients begin to learn cognitive restraint skills, acquiring tools to help them in situations that previously would have converted cravings into overeating.
We are not clueless about how to help individuals with overweight and obesity; when patients feel ashamed or discouraged, I remind them, “Your past is inadmissible, your condition is real and you have never been treated.”
Researcher’s Perspective
Denis Richard PhD
Director of research
Institut universitaire de cardiologie et de pneumologie de Québec (IUCPQ)
Chair, Université Laval’s Research Chair on Obesity
Q. What do we know about how brain networks interact with hormonal signaling and the environment in obesity?
A. Obesity can be thought of as an imbalance between energy intake (food) and energy expenditure (physical activity and thermogenesis). Our obesogenic environment, which markets and offers energy-dense palatable food while encouraging a sedentary lifestyle, is a contributor, as are some genetic factors that are linked to personality traits.
Variations in energy (fat) stores are sensed by the brain and controls on food intake and energy expenditure are adjusted to limit them, as when reduced fat stores cause reduction of the adipocyte-derived hormone leptin. This, in turn, stimulates food intake and reduces energy expenditure to re-establish fat stores at their “defended,” pre-fat loss levels.
Brain networks are involved in energy regulation: activation of the melanocortin system, whose main component is the melanocortin receptor 4 (MC4R), reduces food intake and stimulates energy expenditure, with catabolic effects. We see that loss of function of MC4R is associated with severe obesity in humans as well as in laboratory animals.
Importantly, these energy balance brain networks include brain circuits that also integrate environmental information such as food cues. Such cues, which often represent pleasurable signals in an obesogenic environment, blur the mere homeostatic control of food intake through the influence they have on the reward system.
This largely cognitive system includes a motivational “wanting” circuit, mainly composed of dopamine neurons that constitute the brain mesolimbic pathway. This brain circuit connects the ventral tegmental area to the nucleus accumbens (NAc). The reward system also includes a hedonistic “liking” circuit that includes opioid and endocannabinoid neurons concentrated in the NAc.
The reward system is a very important part of the appetitive network that can contribute to overeating despite being in a sated state, as when one eats a tasty dessert after a satiating main course. Because homeostatic and cognitive (reward and executive) brain circuits are interconnected, changes in energy stores influence the reward and executive systems. Fat loss enhances the rewarding effects of food cues and weakens self-discipline, contributing to the difficulty people with obesity have in maintaining weight loss.
Q. Could you please speak to some of the contributions your research group has made in this field?
A. A primary focus is the neural and hormonal control of food intake and energy expenditure in obesity, which we approach in an integrative manner. We recently published on two emerging brain molecules involved in energy balance: the protein DEPTOR has a link to energy balance regulation DEPTOR (Caron A et al, J Comp Neurol 2015; Caron A et al. Mol Metab 2016). We also recently described the anorexigenic and thermogenic roles of Acyl-CoA binding domain containing 7 and its derived peptide that we term nonadecaneuropeptide (NDN), a significant player in the regulation of energy balance (Lanfray D et al. eLife 2016; Lanfray D, Richard D Front Neurosci 2017).
In the bariatric surgery area, we are currently investigating the effects of biliopancreatic diversion (BPD) on the gut microbiota, demonstrating its striking effect of shifting the microbiota from Clostridiales domination prior to the surgery (or in SHAM rat) to Bifidobacteriales predominance post-surgery (Mukorako P et al, ObesityWeek, Nashville, Nov 2018).
Colleagues and I have also recently undertaken a major research program using functional magnetic resonance imaging to investigate neuroanatomical and neurobehavioral changes after bariatric surgery. Our preliminary results demonstrate that white matter volume is significantly increased 4 months after bariatric surgery (Michaud A et al, ObesityWeek, Nashville, Nov 2018).