Rheumatology
EULAR 2020 eCongress
Selective JAK Inhibitors Tested for Control of Psoriatic Arthritis
Virtual Meeting – Presented virtually as a latebreaker at the 2020 EULAR eCongress, the first of two phase 3 trials of a selective JAK inhibitor has been completed in patients with psoriatic arthritis (PsA). These phase 3 data in PsA, which included a direct comparison to a tumor necrosis factor (TNF) inhibitor, predict a broader array of indications for agents with the selective subclass of JAK inhibitors, two of which were recently approved in Canada for the treatment of rheumatoid arthritis (RA).
Currently, most of the targeted therapies for PsA are injectable monoclonal antibodies that inhibit TNF or other pro-inflammatory cytokines. Tofacitinib, an exception, is a first-generation oral JAK inhibitor approved for PsA, but not all patients respond to any of the available treatments, and many patients with an initial response become refractory over time. The newer oral JAK inhibitors were developed to target the JAK subtypes most closely associated with inflammation.
At the EULAR eCongress, trial data were presented with the JAK inhibitors filgotinib and upadacitinib. A phase 3 trial was presented in patients who had failed or were intolerant to biologic disease-modifying anti-rheumatic drugs (bDMARDs). Data with filgotinib, an experimental selective JAK inhibitor, were derived from EQUATOR2 an open-label extension of the previously completed phase 2 EQUATOR. The data for upadacitinib, which has joined baricitinib with an indication for RA in Canada, were presented in a latebreaker phase 3 trial conducted in patients naïve to targeted therapies.
The data were compelling. In the latebreaker, called SELECT-PsA 1, upadacitinib at the higher of two study doses outperformed the comparator, a TNF inhibitor.
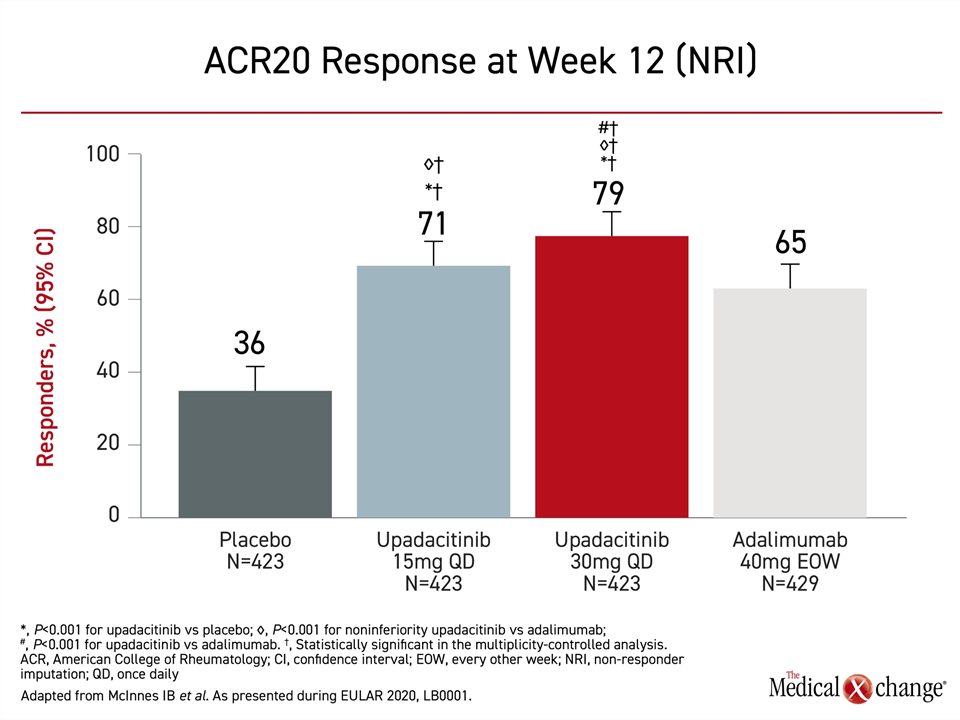
In a population refractory to prior non-biologic conventional synthetic DMARDs (csDMARDs), “the 30 mg once-daily oral dose of upadacitinib achieved superiority to adalimumab,” reported Dr. Iain B. McInnes, Director of the Institute of Infection, Immunity, and Inflammation, University of Glasgow, UK.
“Relative to adalimumab for efficacy, the lower dose of upadacitinib easily met the criterion for non-inferiority, while the higher dose was significant for superiority.”
In the multinational SELECT-PsA-1, 1704 patients were randomized to 15 mg upadacitinib once daily, 30 mg upadacitinib once daily, a standard course of 40 mg adalimumab administered every other week, or placebo. Baseline characteristics, which included a mean BMI of slightly greater than 30 and an average PsA duration of about six years at entry, were well balanced across arms.
For the primary endpoint of ACR20, a composite endpoint that includes a 20% improvement in tender and swollen joints, all three active therapies were significantly more effective than placebo, but both doses of upadacitinib achieved at least a numerical advantage over adalimumab (Figure 1). For the lower dose, the efficacy easily met the criterion for non-inferiority (P<0.001). For the higher dose, the greater response was highly significant for superiority (P<0.001).
A similar hierarchy of response for upadacitinib relative to adalimumab was observed for ACR50 an ACR70. In both cases, the response on the 30 mg dose of upadacitinib was superior (P<0.001) to adalimumab. The numerical advantage for the 15 mg dose of upadacitinib to adalimumab was consistent for these endpoints but not significant.
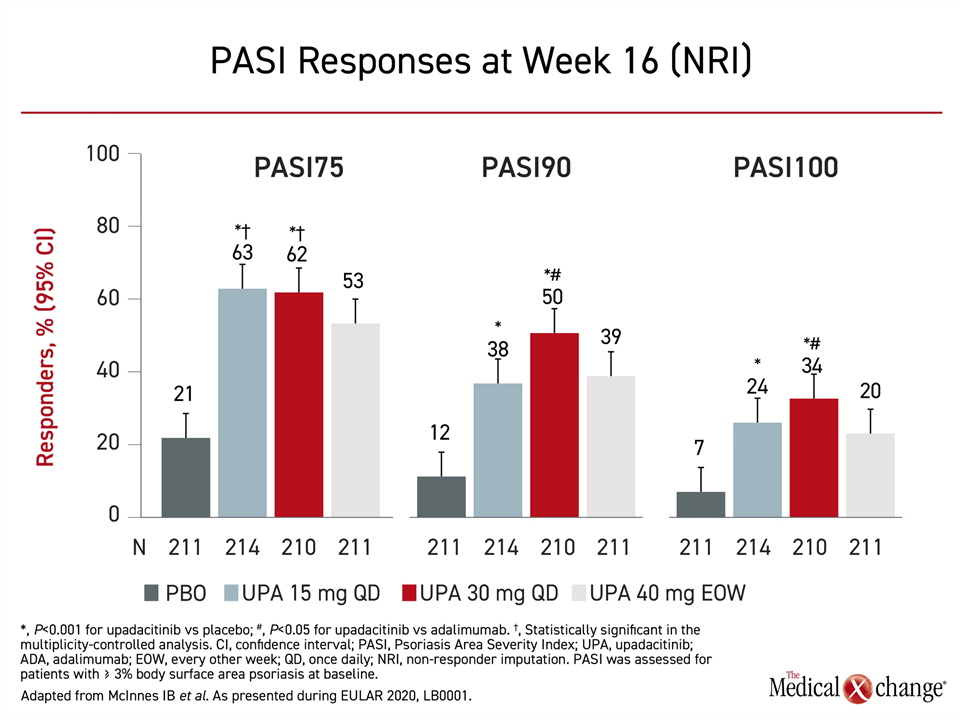
For the skin component of PsA measured with the Psoriasis Area and Severity Index (PASI), the advantage of 30 mg upadacitinib relative to adalimumab for both PASI90 and PASI100 met significance (P<0.05), but the numerical advantage of 15 mg and 30 mg doses of upadacitinib for PASI75 did not (Figure 2).
Minimal Disease Activity Comparison
At week 24, the proportion of patients achieving minimal disease activity was 37% and 45% for the 15 mg and 30 mg doses of upadacitinib, respectively, versus 33% for adalimumab. The difference for the 30 mg dose relative to the TNF inhibitor was statistically significant (P<0.001). The same relative advantage for the higher dose was observed for the Health Assessment Questionnaire – Disability Index (HAQ-DI), the SF-36 quality of life instrument, and a pain scale at Week 12 (all P<0.001). In all cases, the response was higher on the 15 mg dose but not significantly different relative to adalimumab.
About 30% of patients entered the study with dactylitis and 60% with enthesitis. Resolution of dactylitis was achieved in more than 75% of patients on any active therapy. The numerically higher response in the upadacitinib arms relative to adalimumab did not reach significance. For enthesitis, the greater response in the 30 mg upadacitinib arm was significant relative to adalimumab (58% vs. 47%; P<0.05), but the 54% rate of resolution in the 15 mg upadacitinib arm was not.
Adverse Event Rates Differ
The rates of discontinuations for an adverse event were low and about the same for the 30 mg dose upadacitinib relative to adalimumab (5.0% vs. 5.1%), but more patients in the 30 mg upadacitinib arm experienced serious adverse events (6.1% vs. 3.7%) than those treated with adalimumab. The rates of discontinuations were lower in the 15 mg upadacitinib arm than either of the other active treatment arms for both adverse events (3.1%) and serious adverse events (3.1%).
When compared for types of serious adverse events, the difference between the 30 mg upadacitinib dose and adalimumab could be explained by a higher number of serious and opportunistic infections, including a higher rate of herpes zoster (4.3% vs. 0.7%). In a comparative phase 3 study of these two drugs in RA called SELECT-COMPARE, the overall rate of serious and opportunistic infections was similar. In that study, herpes zoster was also more common on upadacitinib, but rates overall were low, most were non-serious, and none involved extra-cutaneous involvement.
Referencing the previous phase 3 studies with upadacitinib in RA, Dr. McInnes said that the findings with upadacitinib in SELECT-PsA-1 “were consistent with its known safety profile, with no new safety risks identified.”
A superior effect for a JAK inhibitor relative to a TNF inhibitor has not been documented previously in a large randomized trial.
The second phase 3 trial of upadacitinib in PsA, SELECT-PsA 2, also presented at EULAR 2020, did not include an active comparator arm, but failure on or intolerance to at least one bDMARD was an enrollment criterion. By baseline characteristics, which did not differ significantly between treatment arms, the mean duration of PsA was approximately 10 years, and the average age was about 53 years. The primary endpoint was ACR20.
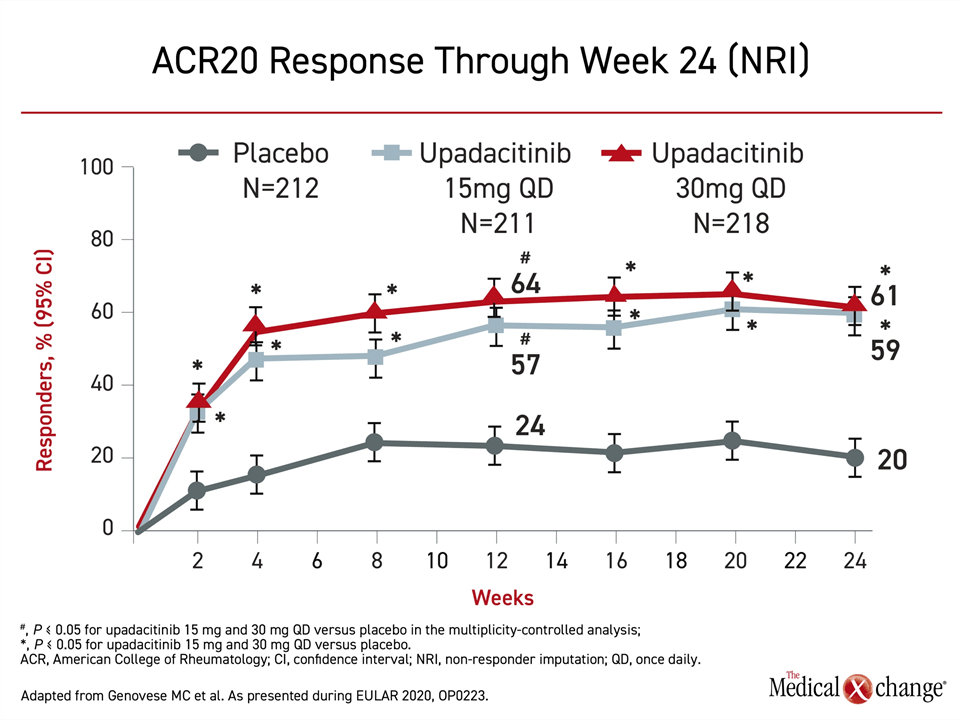
Both the 15 mg and 30 mg doses of upadacitinib were significantly more effective than placebo by two weeks and remained so for the duration of 24 weeks of follow-up, according to Dr. Philip J. Mease, Director of Rheumatology Research, Swedish Medical Center, University of Washington, Seattle (Figure 3). A similar advantage was observed for ACR50 and ACR70.
Activity in Treatment-Experienced PsA Patients
Clinically-meaningful reductions in disease activity were “demonstrated in all core domains,” Dr. Mease reported. He emphasized that the advantage of upadacitinib relative to placebo was seen within two weeks.
“The efficacy of the JAK inhibitor relative to the TNF inhibitor ‘was demonstrated in all core domains.’”
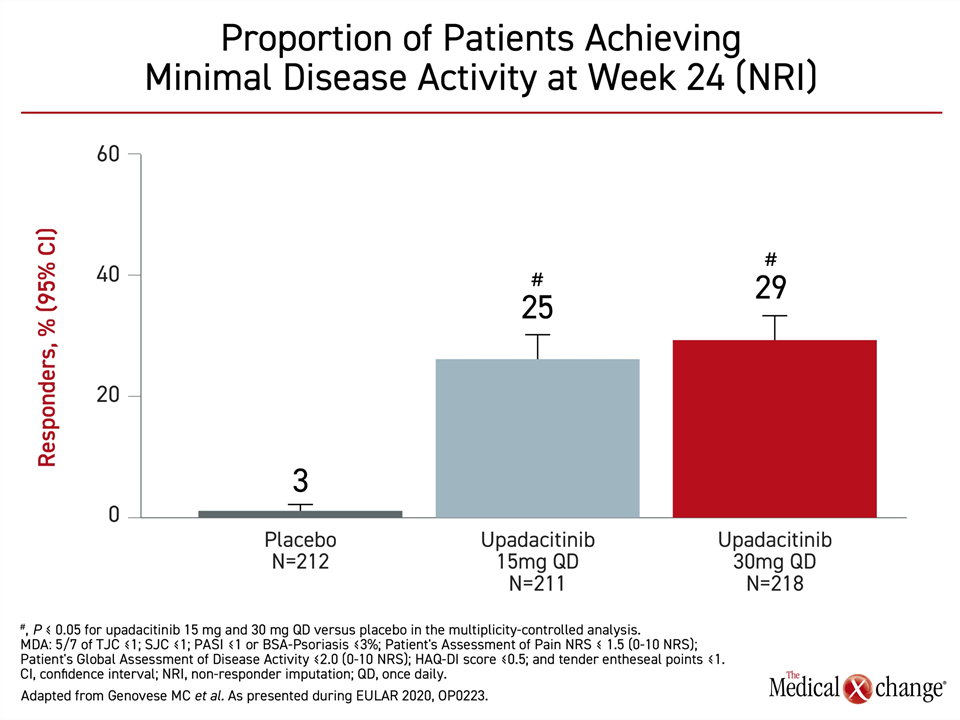
At week 24, minimal disease activity, specifically defined by low scores or absence of symptoms across multiple measures, including PASI and HAQ-DI, were 3%, 25%, and 29%, for the placebo, 15 mg, and 30 mg upadacitinib arms, respectively. The advantage was significant (P<0.05) for both doses of upadacitinib relative to placebo (Figure 4).
Both doses of upadacitinib were also superior to placebo for multiple secondary endpoints evaluated individually. These included HAQ-DI (P<0.05), SF-36 (P<0.05), Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) (P<0.01), Static Investigator Global Assessment (sIGA) of psoriasis (P<0.05) and Self-Assessment of Psoriasis Symptoms (SAPS) (P<0.05).
At baseline about 25% of patients had dactylitis and 65% had enthesitis. At 24 weeks, the dactylitis had resolved in only 28% of those randomized to placebo versus 58% of those in the 15 mg upadacitinib arm and 68% of those in the 30 mg upadacitinib arm (both P<0.05 versus placebo). Enthesitis has resolved in 15% of placebo patients versus 43% and 45% of those in the 15 mg and 30 mg upadacitinib arms (both P<0.05 versus placebo).
Side Effects: 30 mg vs. 15 mg Dose
Adverse events leading to discontinuation of the assigned therapy was higher among those receiving the 30 mg dose of upadacitinib (9.2%) versus either the 15 mg dose of upadacitinib (7.1%) or placebo (5.2%). Serious adverse events were also higher in the 30 mg upadacitinib arm (8.3%) than in the 15 mg upadacitinib arm (5.7%) or the placebo arm (1.9%). Serious infections, which were higher in the 30 mg arm (2.8%) relative to the 15 mg arm and placebo arms (both 0.5%) provides one explanation.
The only venous thromboembolism in the study was observed in a patient randomized to the 15 mg dose of upadacitinib. The only death in the study occurred in the placebo group. Grade 3 or higher laboratory abnormalities, including abnormal liver function and cytopenias, were uncommon in all groups.
Again, as in SELECT-PsA 1, the safety findings of SELECT-PsA 2 “were consistent with the known safety profile of upadacitinib in RA,” according to Dr. Mease. There were “no new safety risks identified.”
“The safety findings of SELECT-PsA 2 ‘were consistent with the known safety profile of upadacitinib in rheumatoid arthritis.’
In the 52-week update of the EQUATOR2 trial with filgotinib, the status of 122 of the 131 patients who participated in the phase 2, 16-week placebo-controlled EQUATOR trial was evaluated in relation to the efficacy and safety of treatment. Patients originally assigned to 200 mg of once daily filgotinib were maintained on this dose while those assigned to placebo were switched to the active therapy.
Of the 11 patients who discontinued therapy by 52 weeks, only three did so for an adverse event. The remaining withdrew consent. From the end of the double-blind phase at 16 weeks, when the ACR20 rate was 86.7% (versus 33.3% in the placebo group), responses were sustained. The ACR20 at 52 weeks was 83.3% among those randomized to filgotinib and 78.6% among those initially assigned to placebo and then switched to filgotinib, reported Dr. Dafna D. Gladman, Professor of Medicine, University of Toronto, Ontario.
Similar sustained response was observed across other measures, including ACR50 and minimal disease activity, she reported. There were no new or unexpected safety signals over the extended follow-up.
Overall, the data in randomized trials suggest substantial clinical benefit with the selective JAK inhibitors in PsA, which have the potential to meaningful expand oral targeted therapies for this disease.
Conclusion
Newer selective JAK inhibitors have demonstrated very high rates of efficacy and acceptable safety for the treatment of PsA in randomized trials. In a phase 3 study with upadacitinib, the 15 mg dose performed at least as well as the injectable TNF inhibitor adalimumab, and the higher 30 mg dose of the oral therapy performed significantly better across multiple endpoints. In an open-label extension of a phase 2 trial with the experimental filgotinib, efficacy was sustained at 52 weeks with no unexpected safety signals. The evidence supports a potential role for these drugs in a challenging disease.