Neurology
Multiple Sclerosis: Expert Review and Commentary from Published Literature
Relapsing-Remitting Multiple Sclerosis: Oral First-line Immunomodulators
Liesly Lee, MD
Associate Professor of Medicine (Neurology)
Sunnybrook Health Sciences Centre
University of Toronto
Toronto, Ontario
Oral immunomodulators are an option in the first-line treatment of relapsing-remitting multiple sclerosis (RRMS) in patients unable or unwilling to tolerate the injectable beta interferons or glatiramer acetate (GA). Management of early RRMS agents can be individualized with these first-line options or when de-escalation is being considered. For most patients, an oral immunomodulator provides a reasonable, effective, safe, and appropriate option.
The Principle of Immunomodulators
Following the diagnosis of multiple sclerosis (MS), the disease course is variable, ranging from extended periods of dormancy to frequent and highly symptomatic relapses associated with accelerated disease progression. High-efficacy therapies, including targeted immunosuppressive agents, have expanded options for those with aggressive disease. For those with early RRMS and milder disease activity or burden, first-line immunomodulators remain a reasonable option for their favorable benefit-to-risk ratio.
Based on progression observed in clinical trials, it has been variably estimated that 10% to 34% of RRMS patients have highly-active disease early in their disease course,1 leaving the majority with intermittent relapses and no immediate sustained progression.
In a survey of 26 studies, 30% of RRMS patients had an Expanded Disability Status Scale (EDSS) score of <3 at 10 years.2 In a Canadian study that followed such a cohort of patients, 52.1% still met this definition when followed for an additional 10 years.3 These are important retrospective observations that are relevant to treatment choices in patients with newly-diagnosed MS. Pragmatically, patients are often treated with first-line agents if one is adopting the “escalation” model, as the ability to predict the rate of progression to disability during the early phase of RRMS can be limited. At this stage, activity in most patients remains low, making effective but well-tolerated agents attractive.4
Eventually, most RRMS patients transition to secondary progressive MS (SPMS) despite treatment.5 The risk of eventual progression provides a rationale for an “induction” model in which early use of potent but often less well-tolerated therapies are used in place of first-line RRMS agents. For this reason, first-line immunomodulators still have remained a common option for RRMS patients who do not have aggressive disease in the early phase.6 However, the arguments for and against escalation versus induction therapy for early management of MS continue to evolve.
Immunomodulators: Oral versus Injectable
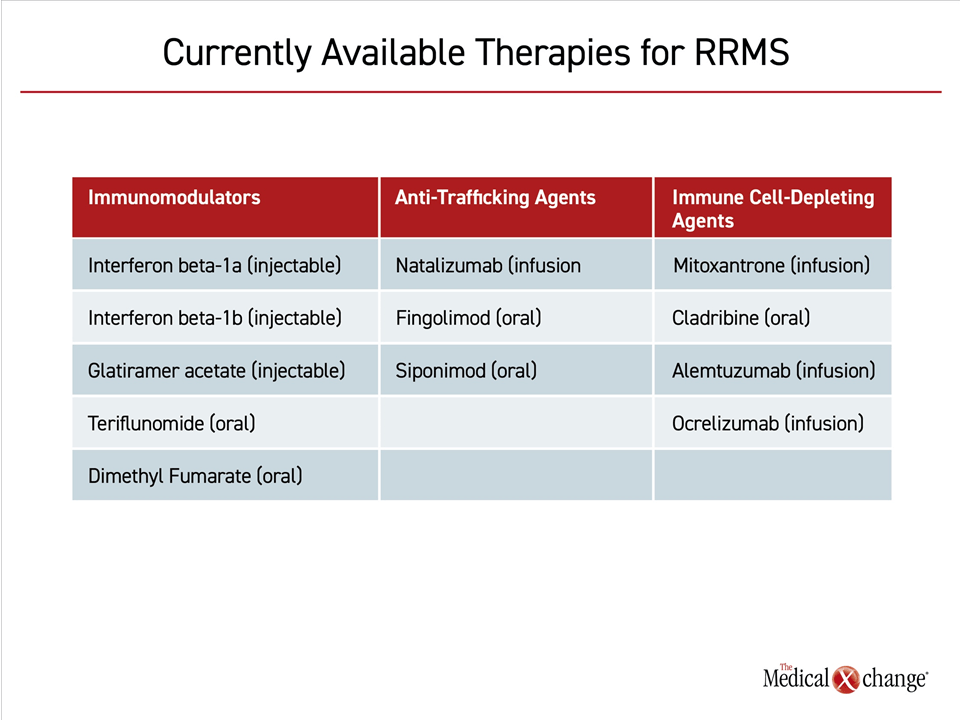
The five immunomodulators currently available for first-line control of RRMS are interferon beta-1b, interferon beta-1a, glatiramer acetate (GA), teriflunomide, and dimethyl fumarate (DMF). The first three of these agents require injection (Table 1). The latter two are oral therapies. In addition to the controlled data from the clinical trials program and the extension studies that followed, ongoing analyses tracking safety and efficacy from a real-world perspective continue to yield evidence of long-term benefit and safety for all of these agents.
There are few direct comparisons of oral and injectable immunomodulators, but indirect comparisons have been attempted using patient matching. In the placebo-controlled phase 3 trial of DMF, otherwise known as the CONFIRM trial, GA was included as a reference comparator.7 In a post-hoc comparison, the twice daily dose of DMF was associated with greater disease control than GA when compared by the annualized relapse rate (ARR) (P=0.02). Two studies employing patient matching techniques also suggested superior efficacy for this oral agent relative to GA on this and other outcomes, such as disease activity on imaging.8,9
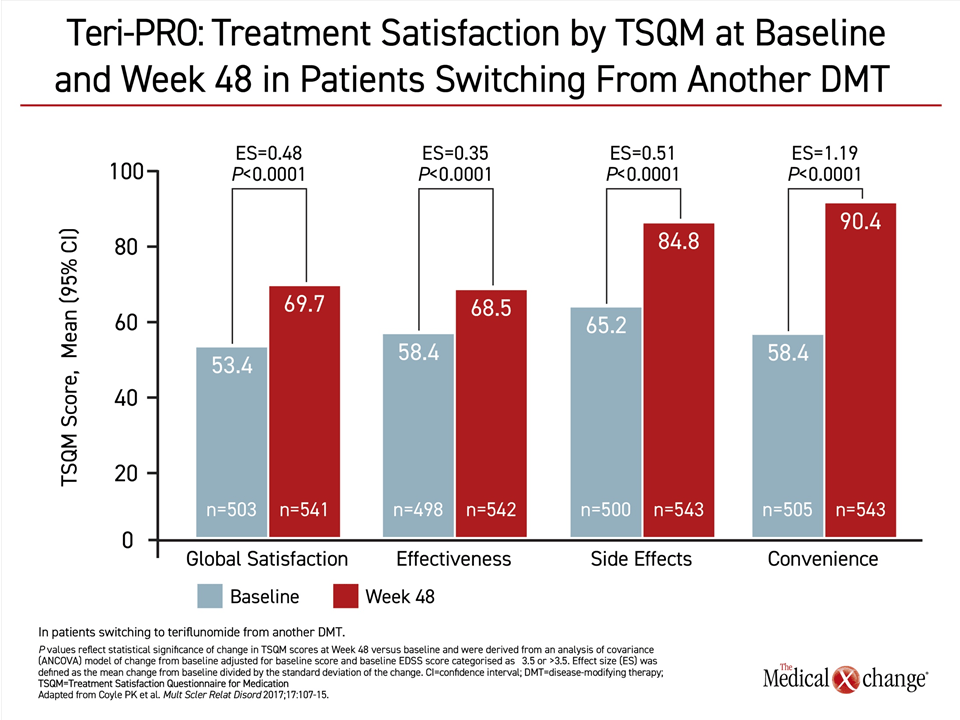
In a study that compared teriflunomide to interferon beta-1a, there was no difference in the 14 mg dose of teriflunomide and the interferon for the composite primary endpoint of time to failure defined as first occurrence of confirmed relapse or permanent treatment discontinuation for any cause.10 There was also no difference in ARR, a secondary endpoint, but teriflunomide was associated with higher score for Treatment Satisfaction Questionnaire for Medication (TSQM). In a phase 4 study called Teri-PRO, 768 RRMS patients who were switched to 14 mg teriflunomide from an injectable disease-modifying therapy were followed for 48 weeks.11 When patient satisfaction was evaluated with the TSQM, there were significant improvements favoring teriflunomide on all four domains evaluated (Figure 1). Of these gains, the largest involved the sense of improved convenience. Based on the evidence that patient satisfaction drives adherence,12 these data provide support for an oral immunomodulator as a reasonable option to injectable drugs as a first-line therapy.
Exceeding antipathy to needles or fear of pain on injection, the inconvenience of regular injections has also been previously linked to non-adherence.13,14 In the STICK study, which evaluated 445 RRMS patients on stable injectable therapy with the TSQM, the average scores across injectable agents were acceptable overall but lower for convenience.15 When asked about alternative strategies for drug delivery, 83% of the participants stated a preference for once-daily oral therapy over other dosing strategies.
However, despite the value some patients place on oral therapies, GA remains a commonly used first-line choice in RRMS. Although comparative data with other first-line therapies remain limited, two reviews of published studies concluded that GA offers comparable protection against relapses relative to the interferons.16,17 Another review characterizing the efficacy of GA relative to DMF for protection against relapses when each was compared to placebo drew a similar conclusion.7 All three reviews cautioned that GA might be less effective for inhibiting disease activity measured with MRI but did not speculate about whether this is meaningful for long-term outcomes. Overall, GA has proven active and well tolerated in more than 20 years of clinical experience. It does not require any special safety monitoring and poses a very low risk of drug interactions. GA is not associated with teratogenicity or an adverse effect on pregnancy outcomes.18 It has been granted a category B pregnancy rating.
RRMS: First-Line Oral Therapies
Teriflunomide and DMF, which were both approved approximately seven years ago on the basis of favorable efficacy and safety in multicenter phase 3 trials, have not been directly compared in a randomized study, but they are now each supported by extensive clinical data. Fingolimod, another oral agent, is considered to be more effective than first-line RRMS therapies,19,20 and is labeled for second-line use in Europe due to a greater relative risk for adverse events.21
The activity of teriflunomide and DMF is attributed to different mechanisms. Teriflunomide inhibits dihydroorotate dehydrogenase, an enzyme important to proliferation of activated T and B cells.22 Clinical benefit appears to be derived from its ability to reduce the number of activated lymphocytes crossing the blood-brain barrier.22 In the phase 3 trials, teriflunomide has been effective whether used in patients who are treatment naïve or had previous exposure to an injectable immunomodulator.23,24 In addition to a high degree of activity relative to placebo for typical clinical endpoints, such as ARR, teriflunomide has also demonstrated protection against brain volume loss and impact on cognitive function at two years.25,26
When compared directly to interferon beta-1a in a randomized trial, teriflunomide in the recommended dose showed comparable efficacy. The numerical advantages for teriflunomide for a composite outcome of time to failure, time to first relapse, or time to treatment discontinuation for any reason did not reach statistical significance.10 However, patient satisfaction scores were higher on the oral therapy than on interferon beta-1a.
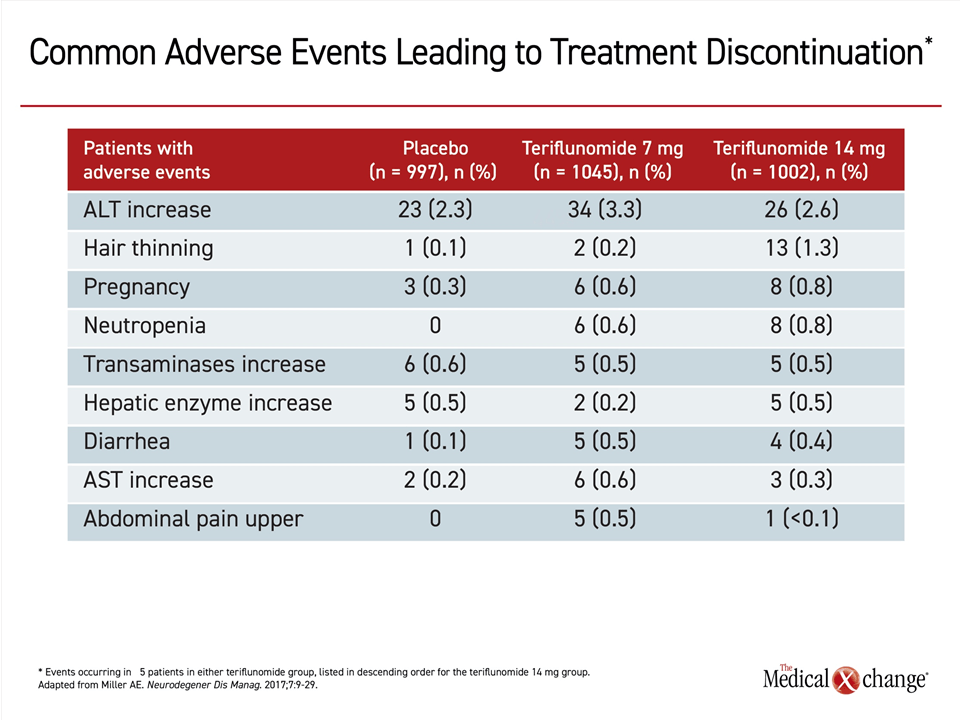
In pooled safety analyses from the placebo-controlled TEMSO and TOWER phase 3 trials, 12.5% of patients discontinued teriflunomide due to an adverse event.27 Elevated liver enzymes accounted for about one third of these discontinuations. Other reasons for discontinuation, such as hair thinning, neutropenia, and diarrhea were uncommon (Table 2). In the long-term extension of TEMSO, with follow-up data extending to nine years,28 there have been no new or unexpected adverse events. Serious and opportunistic infections have been infrequent. The types and rates of malignancies have not deviated from expected patterns.
DMF is an inhibitor of the Nrf2 pathway, which is implicated in the release or proinflammatory cytokines.29 In addition, this agent induces lymphocyte apoptosis, another mechanism that is suspected of playing a role in its therapeutic effect.30,31 In the phase 3 placebo-controlled DEFINE and CONFIRM trials,7,32 a twice-daily dose of 240 mg DMF was associated with a significant reduction in ARR, the number of new or enlarging T2-hyperintense and gadolinium-enhancing lesions at two years. In CONFIRM, DMF was also associated with a reduction in confirmed disability over 12 weeks. The CONFIRM trial included a GA comparator arm that was not part of the randomization. In a post hoc comparison of DMF-treated patients and those in the comparator GA arm, DMF was associated with fewer new or enlarging T2-weighted hyperintense lesions than GA.
There have been no randomized direct comparisons of DMF with other first-line therapies for RRMS, but several retrospective studies have suggested that efficacy is comparable to the interferons, GA, and teriflunomide. In a German registry, ARR rates and time to first relapse compared favorably in those treated with DMF relative to all of these first-line agents.9 Fingolimod, which was included in this analysis, was superior on most of these endpoints to the other agents. Time to therapy discontinuation was similar for all of these agents with the exception of fingolimod, which was longer. In a matching-adjusted indirect comparison of DMF to GA, DMF was found superior for both ARR (P=0.0474) and for 12-week confirmed disability progression (P<0.0001).8 In a retrospective chart review comparing 143 patients receiving interferon to 307 patients receiving DMF, the therapies offered similar efficacy and persistence on treatment.33
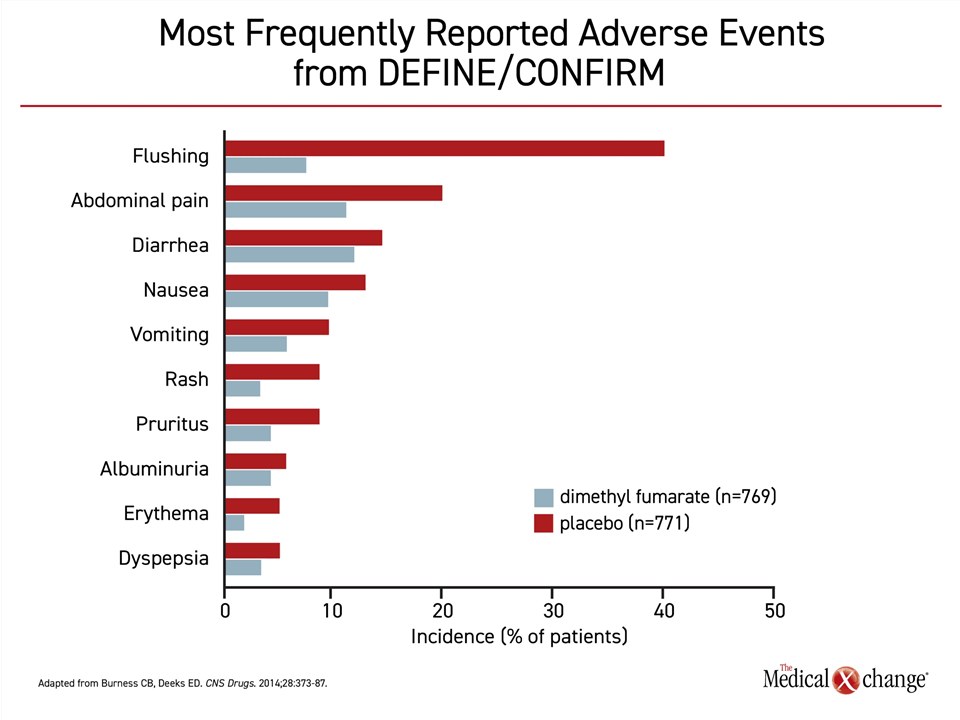
In a pooled safety analysis from the pivotal DMF phase three trials, flushing was identified as the most common side effect. Flushing, occurring in more than 40% of patients, and gastrointestinal side effects were the most common adverse events (Figure 2). 34. However, discontinuations for these events were infrequent. In a five-year analysis of ENDORSE, which is a long-term extension study of patients enrolled in DEFINE and CONFIRM, there have been no new or unexpected adverse events.35 However, there have been rare cases of progressive multifocal leukoencephalopathy (PML) in patients taking DMF. The risk appears to be largely restricted to those exposed to another agent associated with PML or those with prolonged lymphopenia defined as <500 cells/μL.36,37
In the absence of randomized trials, several studies have undertaken comparisons of teriflunomide and DMF using real-world data. These have yielded mixed results. In one study that compared 50 RRMS patients on teriflunomide to 50 patients on DMF across a variety of clinical and MRI endpoints, the drugs were similar for most endpoints although teriflunomide was associated with greater protection against brain atrophy at the end of two years.38 In another, the proportion of patients with at least one new T2 lesion on MRI was lower on DMF, although, again, clinical measures of efficacy did not differ significantly.39 Neither study was randomized and so uncontrolled for issues that affect adherence, such as adverse events or convenience of dosing.
Individualization of Therapy: Making Appropriate Choices
The expansion of therapeutic options has facilitated individualization of therapy. Patient goals and preferences vary and should inform initial and subsequent treatment choices. Although oral immunomodulators are safe and appropriate for first-line treatment, a patient might reasonably prefer an injectable immunomodulator for any of several reasons including freedom from daily dosing. Conversely, a substantial proportion of individuals with mild-to-moderate RRMS might select an oral therapy if it is perceived as better tolerated. Although none of the oral therapies are free of adverse events, these agents have an acceptable long-term safety profile.
For patients with or concerned about risk of progressive disease, the risk of adverse events might be perceived as an acceptable cost for the promise of greater efficacy and the consideration of stronger second-line agents would be reasonable. To aid patients attempting to select the most appropriate immunomodulator, complete information about relative risks not only aids selection but might also improve patient tolerance of adverse events when they occur.
The goal is to guide patients to a therapy with which they can be comfortable. This will be influenced by the patient’s own perspective on convenience and relative risks. Other issues, such as family planning or cost of treatment, might also be relevant. Teriflunomide has been associated with teratogenicity in animals,40 but the risk of adverse pregnancy outcomes has not been confirmed in a population-based study.41 Contraception is therefore advised for patients taking teriflunomide. DMF has not been associated with teratogenicity in animals or adverse outcomes in pregnancy, but it has a class C designation from the U.S. FDA, signifying that there is insufficient data to establish the safety of this agent during pregnancy.42
Neither teriflunomide nor DMF preclude use of subsequent RRMS treatments, including other immunomodulators. Both can be safely used after an injectable immunomodulator. Both can also be considered in a de-escalation strategy in patients with diminished disease activity after a high-efficacy treatment. The concept of early use of high-efficacy therapies, deescalating to first-line immunomodulators remains an intriguing strategy for reducing the risks of high-efficacy agents,43 but the impact on long-term outcomes is unknown.
Summary
Oral immunomodulators can be considered a first-line therapy for RRMS. For once-daily teriflunomide, more than 10 years of safety data from controlled trials and real-world experience show a level of safety and tolerability that rivals that of the injectable immunomodulators. The body of evidence supporting the safety of DMF is comparable. With the exception of rare cases of PML associated with DMF, there has been no pattern of unexpected immune-related events, such as opportunistic infections or malignancies with these oral agents, which are often judged to be more convenient that injectable immunomodulators. Published data suggest they are no less effective for prevention of relapse, reducing disease activity on imaging, or slowing progression to disability.
References
1. Fernandez O. Is there a change of paradigm towards more effective treatment early in the course of apparent high-risk MS? Mult Scler Relat Disord 2017;17:75-83.
2. Reynders T, D’Haeseleer M, De Keyser J, Nagels G, D’Hooghe M B. Definition, prevalence and predictive factors of benign multiple sclerosis. eNeurologicalSci 2017;7:37-43.
3. Sayao AL, Devonshire V, Tremlett H. Longitudinal follow-up of “benign” multiple sclerosis at 20 years. Neurology 2007;68:496-500.
4. Langer-Gould A, Popat RA, Huang SM, et al. Clinical and demographic predictors of long-term disability in patients with relapsing-remitting multiple sclerosis: a systematic review. Arch Neurol 2006;63:1686-91.
5. Gross HJ, Watson C. Characteristics, burden of illness, and physical functioning of patients with relapsing-remitting and secondary progressive multiple sclerosis: a cross-sectional US survey. Neuropsychiatr Dis Treat 2017;13:1349-57.
6. Freedman MS, Selchen D, Prat A, Giacomini PS. Managing Multiple Sclerosis: Treatment Initiation, Modification, and Sequencing. Can J Neurol Sci 2018;45:489-503.
7. Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087-97.
8. Chan A, Cutter G, Fox RJ, Xiao J, Lewin JB, Edwards MR. Comparative effectiveness of delayed-release dimethyl fumarate versus glatiramer acetate in multiple sclerosis patients: results of a matching-adjusted indirect comparison. J Comp Eff Res 2017;6:313-23.
9. Braune S, Grimm S, van Hovell P, et al. Comparative effectiveness of delayed-release dimethyl fumarate versus interferon, glatiramer acetate, teriflunomide, or fingolimod: results from the German NeuroTransData registry. J Neurol 2018;265:2980-92.
10. Vermersch P, Czlonkowska A, Grimaldi LM, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler 2014;20:705-16.
11. Coyle PK, Khatri B, Edwards KR, et al. Patient-reported outcomes in relapsing forms of MS: Real-world, global treatment experience with teriflunomide from the Teri-PRO study. Mult Scler Relat Disord 2017;17:107-15.
12. Barbosa CD, Balp MM, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence 2012;6:39-48.
13. Klauer T, Zettl UK. Compliance, adherence, and the treatment of multiple sclerosis. J Neurol 2008;255 Suppl 6:87-92.
14. Reynolds MW, Stephen R, Seaman C, Rajagopalan K. Persistence and adherence to disease modifying drugs among patients with multiple sclerosis. Curr Med Res Opin 2010;26:663-74.
15. Fernandez O, Duran E, Ayuso T, et al. Treatment satisfaction with injectable disease-modifying therapies in patients with relapsing-remitting multiple sclerosis (the STICK study). PLoS One 2017;12:e0185766.
16. Scott LJ. Glatiramer acetate: a review of its use in patients with relapsing-remitting multiple sclerosis and in delaying the onset of clinically definite multiple sclerosis. CNS Drugs 2013;27:971-88.
17. La Mantia L, Di Pietrantonj C, Rovaris M, et al. Interferons-beta versus glatiramer acetate for relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev 2016;11:CD009333.
18. Sandberg-Wollheim M, Neudorfer O, Grinspan A, et al. Pregnancy Outcomes from the Branded Glatiramer Acetate Pregnancy Database. Int J MS Care 2018;20:9-14.
19. Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402-15.
20. Braune S, Lang M, Bergmann A, NeuroTransData Study G. Efficacy of fingolimod is superior to injectable disease modifying therapies in second-line therapy of relapsing remitting multiple sclerosis. J Neurol 2016;263:327-33.
21. Dorr J, Paul F. The transition from first-line to second-line therapy in multiple sclerosis. Curr Treat Options Neurol 2015;17:354.
22. Bar-Or A, Pachner A, Menguy-Vacheron F, Kaplan J, Wiendl H. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 2014;74:659-74.
23. O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011;365:1293-303.
24. Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014;13:247-56.
25. Sprenger T, Lebrun-Frenay C, Vermersch P, Park MS. Investigation the effect of teriflunomide on brain volume loss in the phase 3 TOPIC study. Neurology 2019;92 (15 supplement):P3.2-046.
26. Sprenger T. Cognitive Outcomes in TEMSO Core Study. European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2017; Paris, France. p. P685.
27. Comi G, Freedman MS, Kappos L, et al. Pooled safety and tolerability data from four placebo-controlled teriflunomide studies and extensions. Mult Scler Relat Disord 2016;5:97-104.
28. O’Connor P, Comi G, Freedman MS, et al. Long-term safety and efficacy of teriflunomide: Nine-year follow-up of the randomized TEMSO study. Neurology 2016;86:920-30.
29. Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011;134:678-92.
30. Li W, Khor TO, Xu C, et al. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol 2008;76:1485-9.
31. Treumer F, Zhu K, Glaser R, Mrowietz U. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol 2003;121:1383-8.
32. Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098-107.
33. Ernst FR, Barr P, Elmor R, Wong SL. Relapse outcomes, safety, and treatment patterns in patients diagnosed with relapsing-remitting multiple sclerosis and initiated on subcutaneous interferon beta-1a or dimethyl fumarate: a real-world study. Curr Med Res Opin 2017;33:2099-106.
34. Burness CB, Deeks ED. Dimethyl fumarate: a review of its use in patients with relapsing-remitting multiple sclerosis. CNS Drugs 2014;28:373-87.
35. Gold R, Arnold DL, Bar-Or A, et al. Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: Interim analysis of ENDORSE, a randomized extension study. Mult Scler 2017;23:253-65.
36. Gieselbach RJ, Muller-Hansma AH, Wijburg MT, et al. Progressive multifocal leukoencephalopathy in patients treated with fumaric acid esters: a review of 19 cases. J Neurol 2017;264:1155-64.
37. Diebold M, Altersberger V, Decard BF, Kappos L, Derfuss T, Lorscheider J. A case of progressive multifocal leukoencephalopathy under dimethyl fumarate treatment without severe lymphopenia or immunosenescence. Mult Scler 2019;25:1682-5.
38. Zivadinov R, Kresa-Reahl K, Weinstock-Guttman B, et al. Comparative effectiveness of teriflunomide and dimethyl fumarate in patients with relapsing forms of MS in the retrospective real-world Teri-RADAR study. J Comp Eff Res 2019;8:305-16.
39. Laplaud DA, Casey R, Barbin L, et al. Comparative effectiveness of teriflunomide vs dimethyl fumarate in multiple sclerosis. Neurology 2019;93:e635-e46.
40. Cada DJ, Demaris K, Levien TL, Baker DE. Teriflunomide. Hosp Pharm 2013;48:231-40.
41. Andersen JB, Moberg JY, Spelman T, Magyari M. Pregnancy Outcomes in Men and Women Treated With Teriflunomide. A Population-Based Nationwide Danish Register Study. Front Immunol 2018;9:2706.
42. Gold R, Phillips JT, Havrdova E, et al. Delayed-Release Dimethyl Fumarate and Pregnancy: Preclinical Studies and Pregnancy Outcomes from Clinical Trials and Postmarketing Experience. Neurol Ther 2015;4:93-104.
43. Stankiewicz JM, Weiner HL. An argument for broad use of high efficacy treatments in early multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2020;7.