Neurology
7th Congress of the European Academy of Neurology (EAN) - Virtual 2021
Head-to-Head Trial Shows Large Clinical Advantage for CGRP Monoclonal Antibody in Migraine Prophylaxis
Virtual Meeting – In a head-to-head, double-blind, double-dummy trial, an antagonist of the calcitonin gene-related peptide (CGRP) receptor was more effective and better tolerated than a traditional and commonly used standard therapy for the prevention of migraine. Following approval of the first CGRP inhibitor in 2018, the clinical experience over the past three years predicted the promising results of this study. Yet, the substantial advantages help quantify the relative advantage of this targeted therapy for episodic and chronic migraine, a condition for which there is an urgent need for preventive therapies with greater efficacy and tolerability.
CGRP, a neuropeptide, was identified as a target for migraine prophylaxis on the basis of experimental and clinical studies in which it was confirmed as a mediator of migraine. These studies led to the development of CGRP monoclonal antibodies as the first targeted treatment of migraine. Erenumab, a monoclonal antibody that targets the CGRP receptor, was approved in 2018. Related agents have followed. At the 2021 EAN, potentially practice-changing data from a direct comparison trial, called HER-MES, were presented as a latebreaker. No multicenter controlled trial has previously compared a CGRP monoclonal antibody to a standard treatment for migraine prophylaxis.
First Head-to-Head Migraine Prevention Trial
“As the first head-to-head trial to compare a therapy targeting the CGRP receptor to a standard-of-care option for migraine prophylaxis, HER-MES informs clinical decision-making,” said Dr. Uwe Reuter, Department of Neurology, Charité University Hospital, Berlin, Germany. Dr. Reuter, the lead author of HER-MES, explained that these data provide an opportunity to gauge the clinical yield of erenumab over non-specific therapy on the basis of safety, efficacy, and quality of life.
“This first head-to-head trial of a CGRP monoclonal antibody and a standard-of-care informs clinical decision-making for migraine prophylaxis.”
In the HER-MES trial, erenumab was compared to topiramate, an oral anticonvulsant that has been previously associated with efficacy for migraine prevention in several studies including a multicenter double-blind, placebo-controlled trial (Brandes JL et al. JAMA 2004;291:965-973). Although the mechanism by which topiramate prevents migraine is not well understood, it is among the most common pharmacologic options employed for this indication. One limitation of this and many other standard but non-specific therapies for long-term prophylaxis is high rates of discontinuation due to tolerability issues.
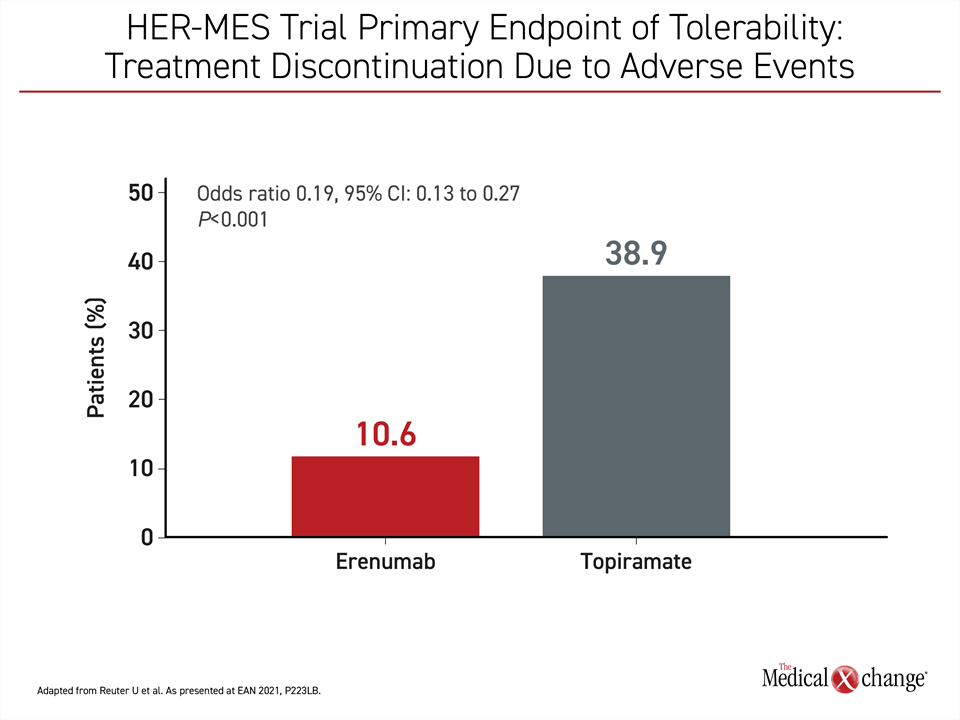
Primary Endpoint: Treatment Discontinuation
Due to the importance of tolerability for effective migraine prophylaxis for otherwise active therapies, treatment discontinuation due to adverse events was selected as the primary endpoint of the HER-MES trial. For comparing efficacy, the secondary endpoint was at least a 50% reduction from baseline in monthly migraine days (MMD). Change in quality of life, another predefined endpoint, was measured with the six-item Headache Impact Test (HIT-6) and the 36-item Short Form Health Survey (SF-36).
Employing a double-blind, double-dummy design, the trial randomized 777 patients at 82 participating sites in Germany. All patients took a daily pill, whether topiramate or placebo, and received a monthly injection, whether erenumab or placebo. Outcomes were assessed at the end of 24 weeks.
At the end of six months, 38.9% of those randomized to topiramate versus 10.6% of those randomized to erenumab had discontinued therapy due to an adverse event. By odds ratio (OR), this translated into a more than 80% reduction in risk of discontinuation over the period of study (OR 0.19; P<0.001) (Figure 1). Although serious adverse events were low in both groups (0.5% for topiramate vs. 0.3% for erenumab), the absolute number of treatment-related adverse events of any kind were higher on topiramate (81.2% vs. 55.4%).
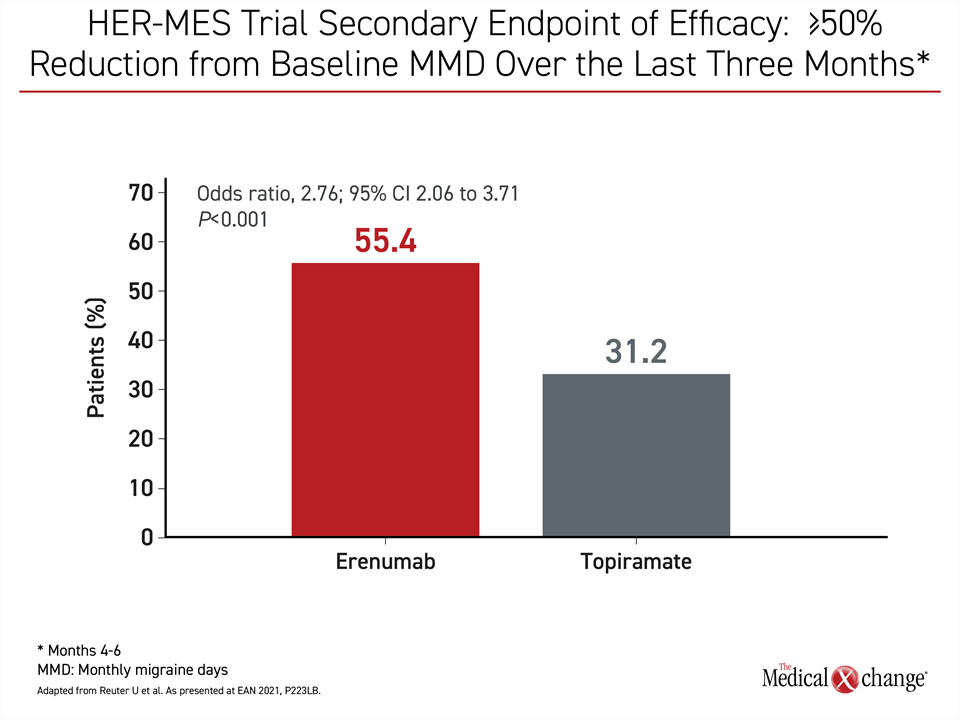
Efficacy Achieved by 55.4% vs. 31.2%
The efficacy endpoint of ≥50% reduction in MMD over the last three months of the study was achieved by 31.2% of those randomized to topiramate versus 55.4% of those randomized to erenumab. Again, the difference favoring erenumab was highly significant (HR 2.76; P<0.001) (Figure 2).
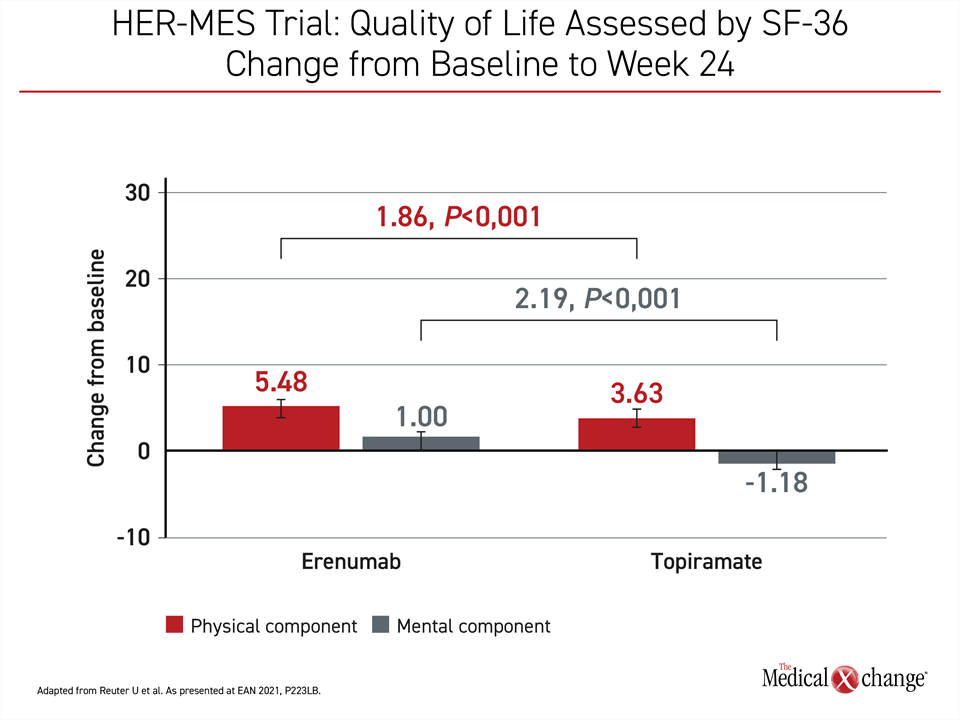
The clinical significance of these differences is reflected in quality of life comparisons. Topiramate achieved a 7.72-point reduction in HIT-6 score at the end of study relative to baseline. With a reduction of 10.9 points, the absolute gain of 3.16 points in the erenumab group produced a substantial statistical and clinical advantage (41%; P<0.001). On SF-36, there was also a large relative gain for erenumab over topiramate from baseline in both the physical and mental components. On the physical component, the absolute gain represented a 50% improvement (Figure 3).
At baseline, the patient population in both arms of the HER-MES trial was typical of candidates for prophylactic migraine therapy and well matched. About 85% were female. The mean age was 41 years old, although it included study participants up to the age of 66 years. The mean history duration of recurrent migraine was nearly 22 years with a monthly baseline MMD of 11.5 days. Nearly 10% had an average of 15 days or more of migraine per month. Most of the others had 8 to 14 days of migraine per month.
At the time of study entry, more than 80% of patients were routinely using treatments to control migraine symptoms, but none were taking prophylactic therapy. Of patients, nearly 60% were naïve to prophylactic therapy. Most of the remainder had failed one prior prophylactic regimen, but about 10% had failed two or more.
Uptitration of Comparator Permitted
After randomization, patients entered a six-week blinded titration phase. For those randomized to topiramate, an increase in dose of up to 100 mg from the starting dose of 50 mg was permitted as tolerated. Erenumab was administered subcutaneously in a dose of 70 mg or 140 mg at the discretion of the treating investigator. Safety and efficacy were compared from week 6 to the end of 24 weeks. Patients continued to be monitored for safety once monthly following the study conclusion.
“Prophylactic medicine is employed by only a small proportion of the patients of those most affected by migraine.”
The advantage of the targeted erenumab therapy over topiramate has major implications for reducing the burden of migraine, according to Dr. Reuter. Even though migraine has been identified as one of the most common non-fatal causes of absenteeism and impaired activity of daily living, prophylactic medicine is employed by only a small proportion of the patients qualifying for prevention.
In a study of migraine burden in the United States, only 13% of patients considered to be candidates were taking preventive therapy. In some cases, patients had never been prescribed prophylaxis, but the proportion of patients who had once received prophylaxis but stopped were also substantial. Although Dr. Reuter explained that patients frequently discontinue treatment due to perceived lack of efficacy, these therapies are “discontinued largely due to tolerability issues.”
Obstacles to Sustained Adherence
For preventative therapies in general and for migraine specifically, tolerability is a critical variable. Independent of the challenge of remembering to take preventative therapy on non-symptom days, adverse events provide an incentive to forego preventive treatments in the absence of an acute need. The potential for incomplete adherence to create the opportunity for migraine recurrence is the basis of a vicious cycle of inadequate symptom control and discouragement about the efficacy of prophylaxis.
The tolerability of erenumab relative to previous therapies is attributed to its target action on CGRP signaling, which activates the trigeminovascular system. Off-target effects, such as constipation, have been reported, but significant issues have been uncommon and largely self-limited. As a monthly injection, this therapy further ensures steady drug levels and thereby circumvents the risk of partial adherence to daily oral therapies.
At the EAN, data from an open-label study of 214 patients who have been followed on five years of continuous erenumab prophylaxis support persistent benefit and long-term safety. The most common adverse events over this period of follow-up, such as nasopharyngitis, influenza, and upper respiratory tract infections, were not likely to be treatment related, according to author Dr. Massoud Ashina, Danish Headache Center, University of Copenhagen, Denmark.
No New Side Effects Over 5 Years
“There was no emergence of new or unexpected side effects over time,” said Dr. Ashina, who reported that the 214 patients followed for five years are from an initial enrollment of 383 patients. Much of the attrition over the course of follow-up was due to lost follow-up, personal decisions, symptom abatement, or other reasons not related to efficacy or adverse events.
In general, “there were consistent and sustained clinical responses among the patients who completed all five years on therapy,” Dr. Ashina said, noting disease control at the end of study was similar to that observed immediately after erenumab was initiated.
“There were consistent and sustained clinical responses among the patients who completed all five years on therapy.”
“The majority of patients continued to achieve at least a 50% reduction in monthly migraine days from baseline,” he reported. Seventy-four percent of patients maintained at least a five-point reduction in HIT-6 score at the five-year follow-up.
Real-World Tracking of CGRP Monoclonal Antibody Use
Several ongoing real-world studies tracking the safety and efficacy of erenumab, including SQUARE, which is following migraineurs in Switzerland and IMPROVE, which is tracking patients at 10 centers in Denmark, Sweden, and Norway, are generating similar data. Preliminary data from the IMPROVE study, which has so far enrolled 159 patients followed for at least six months, has associated erenumab with a 42% reduction in MMD from baseline (from 17.4 MMD to 10.2 MMD at 6 months) and a 10% reduction in the HIT-6 score (59.5 vs. 66 points) (Amin FM et al. As presented at EAN 2021, EPO-735).
“The patients enrolled in IMPROVE have typically failed multiple migraine preventive strategies,” said Dr. Faisal Mohamed Amin, Danish Headache Center, University of Copenhagen, Denmark.
In a retrospective registry study conducted in Finland, the objective was to compare the number of sick-leave days and the number of medical-facility visits due to headache in the 12 months after initiating erenumab relative to the prior 12 months. The 82 patients included in this analysis had all received a minimum of two monthly doses of erenumab.
Sick-Leave Days Reduced 74%
“Treatment with erenumab reduced headache-related sick-leave days by 74% and headache related visits by 45%,” reported Dr. Markku Nissilä, Chief Medical Officer, Terveystalo Biobank and Clinical Research, Turku, Finland. For impact on sick-leave days, this involved a statistically significant reduction from 5 days in the year prior to treatment to 1.3 days after therapy (Nissilä M et al. As presented at EAN 2021, A-21-01058).
“Treatment with erenumab reduced headache-related sick-leave days by 74% and headache related visits by 45%.”
The substantial clinical burden imposed by chronic and episodic headache has long argued for prophylaxis, but this strategy is employed in low rates of patients due to the limited efficacy and off-target effects of non-specific therapies. According to Dr. Reuter, targeted therapies provide an opportunity for efficacy, acceptable tolerability, and rapid onset of action. He suggested the head-to-head data are of particular value for determining when to use erenumab relative to current standards of care.
Conclusion
The head-to-head HER-MES trial is the first objective controlled trial to compare a targeted CGRP monoclonal antibody to a commonly used non-specific standard-of-care comparator to prevent migraine episodes in patients with frequent attacks. At the end of six-months, the discontinuation rate was 80% lower on the targeted anti-CGRP therapy. For the endpoint of at least a 50% reduction in migraine days from baseline, the efficacy was nearly 50% greater with erenumab. Long-term data show consistent benefit and no increased risk of side effects over five years of maintenance therapy.