hematology
63rd American Society of Hematology (ASH) Annual Meeting and Exposition
BTK Inhibitor Paradigm Shift in Waldenström Macroglobulinemia: Clinical Implications
Atlanta – Data with a second-generation Bruton’s tyrosine kinase inhibitor (BTKi) are driving a paradigm shift for treatment of Waldenström macroglobulinemia (WM). The pivotal WM trial with a first-generation BTKi was widely considered practice changing, but subsequent studies have demonstrated that BTK specificity is important. In WM, this was confirmed more than a year ago in a phase 3 head-to-head trial comparing a first- to a second-generation BTKi. At the 2021 ASH annual meeting, an intolerance study with the same second-generation BTKi reinforced the advantage of avoiding off-target effects.
The pivotal trial with the first-generation BTKi ibrutinib conducted in treatment-naïve and relapsed/refractory patients with WM, was considered a paradigm shift by identifying an effective oral therapy (Treon SP et al. N Engl J Med 2015;372:1430-1440). Following a subsequent head-to-head comparison with ibrutinib in the phase 3 ASPEN trial (Tam CS et al. Blood 2020;136:2038-2050), the second-generation BTKi zanubrutinib, which was associated with robust activity and better tolerability, has joined ibrutinib as an approved therapy for WM and demonstrated an advantage for greater specificity against BTK.
For an exclusive interview with Dr. Christine I. Chen on the impact to clinical practice, click here
The promising tolerability results seen in ASPEN are newly reinforced by data drawn from an ongoing phase 2 intolerance study called BGB-3111-215, presented at the 2021 ASH meeting. Led by Dr. Mazyar Shadman, Fred Hutchinson Cancer Research Center, University of Washington, Seattle, the study showed most patients have sustained response on zanubrutinib after failing to tolerate ibrutinib or another second-generation BTKi, acalabrutinib. The safety benefit, attributed to fewer off-target effects, reaffirms relative specificity as a driver of both drug benefit and safety.
Differences in Adverse Events among BTKi
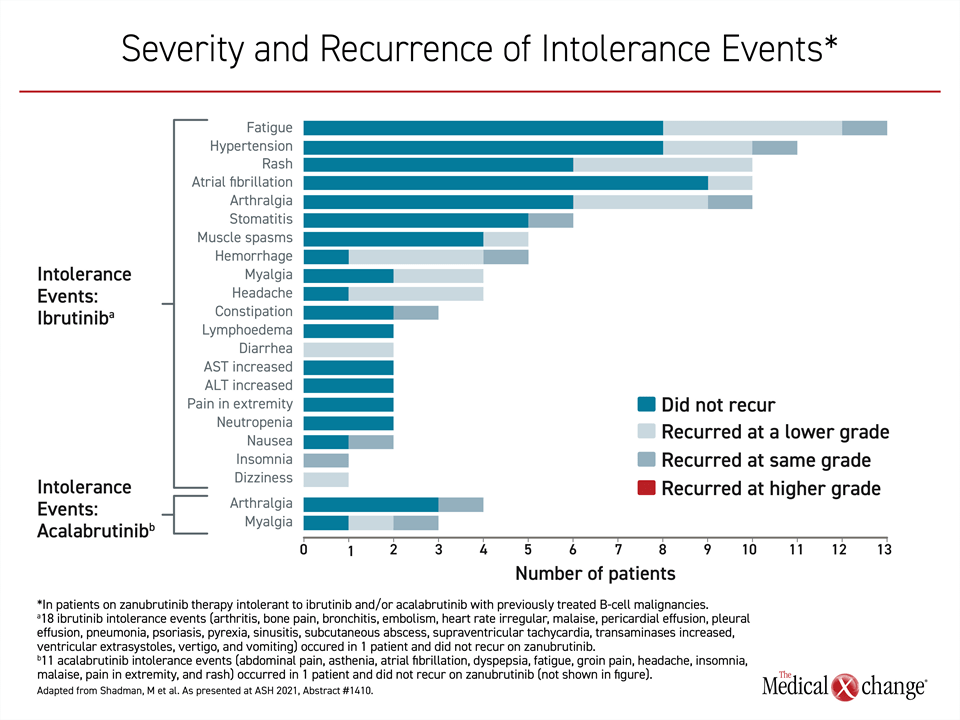
“With a median follow up of 12 months [after being treated with zanubrutinib], 70.4% of ibrutinib intolerance events and 83.3% of acalabrutinib intolerance events have yet to recur while on zanubrutinib,” Dr. Shadman reported.
These data are consistent with the relative specificity of these three BTKi agents. In a study that compared their inhibitory activity against 370 kinases employing a standardized measure of inhibitory concentration, ibrutinib inhibited 17 kinases, acalabrutinib inhibited 15 kinases, but zanubrutinib inhibited just 7 kinases. According to data presented by Dr. Shadman, the greater on-target activity relative to off-target activity of zanubrutinib when compared to other currently available BTKi is likely to explain the lower rate of adverse events associated with this more selective BTKi. The reduced effect on off-target kinases explains the lower rate of adverse events, according to Dr. Shadman.
Relative to the first-generation BTKi ibrutinib, zanubrutinib was “designed to optimize selectivity and maximize BTK occupancy,” Dr. Shadman said. This has been an unmet need. Cardiotoxicity is one of the adverse events that has limited the use of ibrutinib in a number of patients with WM.
Less Specific BTKi Limited by Adverse Events
“BTK inhibition provides effective treatment to several B-cell malignancies [but] the duration of treatment is limited by adverse events leading to treatment discontinuation,” Dr. Shadman pointed out.
Dr. Shadman shared 12-month outcomes from the ongoing intolerance trial in the first 67 patients enrolled. This consists of one cohort of 57 patients who had discontinued ibrutinib and a second cohort of 10 patients who had discontinued acalabrutinib or both ibrutinib and acalabrutinib. All discontinuations from prior BTKi therapy were due to adverse events.
“BTK inhibition provides effective treatment [but] the duration of treatment is limited by adverse events leading to treatment discontinuation.”
Among key inclusion criteria, patients had to have a grade ≥2 non-hematologic toxicity to the prior BTKi for >7 days or a grade ≥3 non-hematologic toxicity for any duration.
The enrollment has not been restricted to those with WM. Rather, other candidates for BTKi are being followed after being treated with zanubrutinib. This includes patients with mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), chronic lymphocytic leukemia (CLL) and small lymphocytic leukemia (SLL). Patients with WM represent about 16.4% of the population.
Promising Tolerability Results after other BTKi Discontinuations
After a median follow-up of 12.0 months with a maximum zanubrutinib exposure of 20.3 months, 84.2% of those who had been intolerant to ibrutinib alone and 80% of those intolerant to acalabrutinib alone or both acalabrutinib and ibrutinib remained on therapy. The median time on the prior BTKi in cohort 1 was 10.6 months vs only 3.3 months in cohort 2. As for the on-study dosing regimen, 63% of patients received zanubrutinib in a dose of 160 mg twice daily and the remainder received 320 mg once daily.
“Of the intolerance events that recurred, 76.5% of those associated with prior ibrutinib and 33.3% of those associated with prior acalabrutinib recurred at same severity,” Dr. Shadman reported. “None recurred at a higher severity” (Figure 1).
Of the more than 80% of patients who have remained on zanubrutinib at 12 months after discontinuing ibrutinib, acalabrutinib, or both, the more specific BTKi “was effective in at least maintaining response in 93.8% or improving response from baseline in 64.1%,” Dr. Shadman said. Only one patient discontinued zanubrutinib due to recurrence of a prior intolerance event on another BTKi. In total, 7.5% of patients discontinued zanubrutinib due to side effects by the end of follow-up.
Intolerance Trial Advantages Consistent with ASPEN
The relative ability of patients to tolerate zanubrutinib after discontinuing ibrutinib or acalabrutinib was similar across the types of B-cell malignancies permitted in this phase 2 trial. The improved tolerability of zanubrutinib relative to ibrutinib is consistent with the findings of the randomized head-to-head ASPEN trial. The senior author of that trial, Dr. Constantine S. Tam, Director of Hematology, St. Vincent’s Hospital, University of Melbourne, Australia, reported that in addition to BTK specificity, pharmacokinetics and pharmacodynamics of zanubrutinib also predict that zanubrutinib has the potential to be both more efficacious and safer than ibrutinib.
In the phase 3 ASPEN trial, 164 patients with relapsed WM (≥1 prior line of therapy) and 37 treatment-naïve WM patients were randomized at 58 study sites to either ibrutinib or zanubrutinib. All patients had symptomatic measurable disease and were eligible for treatment by consensus criteria. The primary efficacy endpoint was a complete response (CR) plus very good partial response (VGPR). The therapies were compared for exposure-adjusted adverse events as well as overall rates of adverse events.
“We observed several clinically-significant differences in the safety and tolerability profiles of the two BTKi, likely consistent with the higher degree of selectivity of zanubrutinib for BTK versus off-target kinases,” Dr. Tam reported.
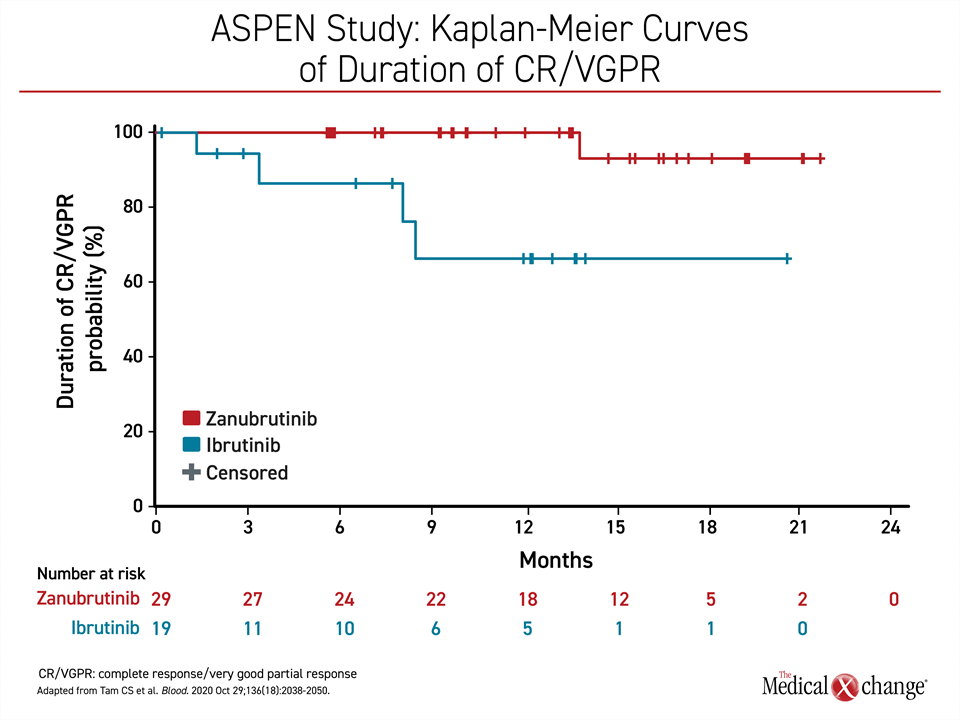
After a median follow-up of 19.4 months, no CRs were identified in either arm of the study, but the increased VGPR rate with zanubrutinib (28% vs. 19%; P=0.09) approached statistical significance. The greater VGPR was observed for zanubrutinib in comparison to ibrutinib in both the relapsed group (29% vs. 20%) and the treatment-naïve group (26% vs. 17%). When plotted over time, the duration of the major responses was greater with zanubrutinib (Figure 2).
The differences between the two BTKi are “likely consistent with the higher degree of selectivity of zanubrutinib for BTK versus off-target kinases.”
The progression-free survival (PFS) rates at 18 months and the duration of major responses did not differ significantly. However, there appeared to be an advantage for zanubrutinib in the event-free rate of the primary combined endpoint of CR and VGFR at 18 months in the relapsed WM group (90% vs. 64%) and the treatment-naïve group (100% vs. not yet determined).
Trend Seen in Adverse Events
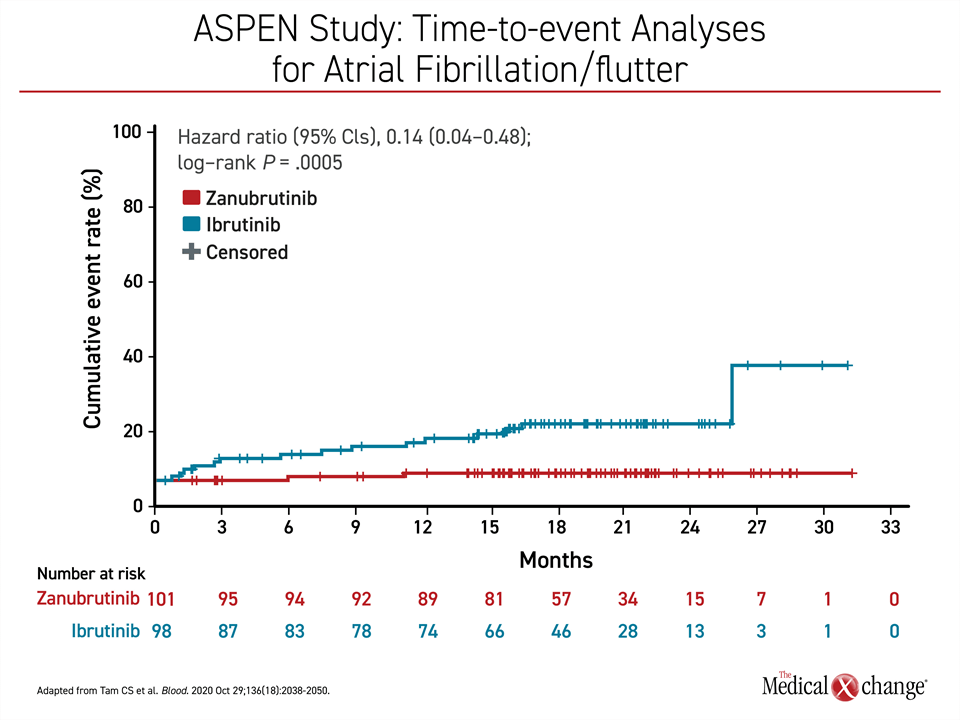
The differences in the side-effect profile of the two BTKi were also an important outcome of the ASPEN trial. While grade 3 or higher adverse events were only modestly higher in the ibrutinib group (63% vs. 58%), atrial fibrillation, diarrhea, muscle spasms, and pneumonia all occurred at rates that were at least 10% higher in the ibrutinib group. Neutropenia was the only adverse event that occurred at a rate 10% or higher in the zanubrutinib group, however, this did not lead to an increase in infection rates.
Many differences in adverse events reached statistical significance. Most notably, the rates of atrial fibrillation or flutter was also significantly higher on ibrutinib (P=0.0005) (Figure 3). This is consistent with prior clinical experience with ibrutinib. Cardiotoxicity has been an important reason for discontinuation of this therapy. In ASPEN, the event rate of any grade atrial fibrillation per 100 person-months was 0.1 in the zanubrutinib group. There was no grade 3 cardiotoxicity versus 0.2 events/100 patient-months in the ibrutinib arm.
Although the rate of infections overall and the rate of grade ≥3 infections were similar in the two groups, neutropenia was more common on zanubrutinib (P=0.007). Pneumonia, in contrast, was more common on ibrutinib (P=0.004). Hemorrhage of any grade was significantly less common on zanubrutinib (P=0.04), but the lower rate of major hemorrhage did not reach statistical significance. At about 15 months, rates of hypertension climbed and then remained higher on ibrutinib relative to zanubrutinib although the difference by the end of the study had not yet reached significance (P=0.16).
The Role of the MYD88 Gene in WM
A mutation on MYD88, which occurs in more than 90% of WM patients, is considered the major source of BTK activation. This might explain why patients with WM without the MYD88 mutation have a poor response to ibrutinib, but this limitation is not shared by zanubrutinib, according to a now published ASPEN substudy (Dimopoulos M et al. Blood Adv 2020;4:6009-6018). In a cohort of 28 patients without the MYD88 mutation or with unknown MYD88 status, labelled as wild-type (MYD88WT), 27% achieved a VGPR and 50% had a major response defined as a partial response or better when treated with zanubrutinib. In limited data with ibrutinib in patients with MYD88WT WM, significant response has been uncommon.
“Our study provides clear evidence that single-agent zanubrutinib is effective in WM patients with MYD88WT,” reported Dr. Meletios Dimopoulos, Chairman, Department of Clinical Therapeutics, National and Kapodistrian University of Athens, Greece. First author of the ASPEN substudy, he reported that WM patients with MYD88WT have been a challenging patient population due to their poor response to most therapies, not just ibrutinib.
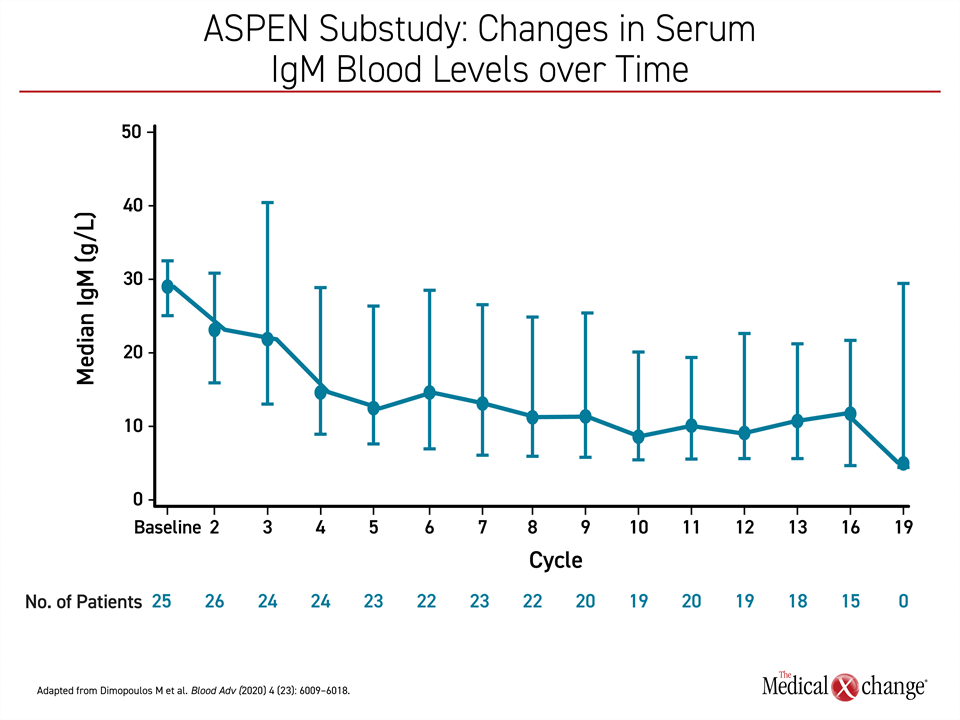
It is unclear as to why zanubrutinib appears to be more active than ibrutinib in MYD88WT WM, but Dr. Dimopoulos observed that other differences in clinical effect of these agents have been observed. Most notably, zanubrutinib was associated with greater and more sustained reductions in IgM than ibrutinib in ASPEN, a finding that might also be relevant to its clinical activity (Figure 4).
“Given that IgM overproduction is the hallmark of WM, the ability to reduce IgM provides an additional efficacy metric with which to evaluate BTKi,” agreed Dr. Tam. “In this regard, results of 2 separate analyses demonstrated significantly deeper and more sustained reductions with zanubrutinib versus ibrutinib.”
“Given that IgM overproduction is the hallmark of WM, the ability to reduce IgM provides an additional efficacy metric with which to evaluate BTK inhibitors.”
In a separate phase 1/2 trial with zanubrutinib, for which extended follow-up recently showed a PFS rate of 80.5% and overall survival rate of 84.8% at 3 years (Trotman J et al. Blood 2020;136:2027-2037), also showed efficacy in MYD88WT WM. Of the 8 WM patients without the MYD88 mutation, 5 (62.5%) achieved a major response including one CR. In other words, the data suggest zanubrutinib is an appropriate choice regardless of MYD88 status.
Conclusion
New data evaluating the tolerability of the second-generation BTKi zanubrutinib in patients with Waldenström macroglobulinemia build upon evidence of the importance of BTK specificity. Following treatment in patients who were intolerant to less-specific BTKi, nearly 65% of patients had an improved response and after one year of follow-up, a majority of intolerable adverse events did not recur while on zanubrutinib. These results are consistent and reinforce those seen from ASPEN, the largest phase 3 trial of BTKi in WM to date, which associated the greater BTK specificity of zanubrutinib with a more favourable benefit-to-risk ratio.