Oncology
Breast Cancer: Expert Review and Commentary from Published Literature
MONALEESA-2 Trial: Milestone for Survival in HR+/HER2- Advanced Breast Cancer
Karen A. Gelmon, MD, FRCPC
Professor of Medicine, University of British Columbia
Medical Oncologist, BC Cancer
Chair UBC/BC Cancer Research Ethics Board
Vancouver, British Columbia
Nathaniel Bouganim, MD, FRCPC
Investigator, RI-MUHC, Glen site Cancer Research Program, Centre for Innovative Medicine
Assistant Professor, Gerald Bronfman Department of Oncology,
Faculty of Medicine and Health Sciences, McGill University
Department of Medicine, Division of Medical Oncology, MUHC
Montreal, Quebec
A new study, MONALESSA-2, has set a new milestone for median survival in postmenopausal women with advanced or metastatic hormone receptor positive and human epidermal growth fact negative (HR+/HER2-) breast cancer. For those randomized to a combination of the cyclin dependent kinase (CDK) 4/6 inhibitor ribociclib and the aromatase inhibitor letrozole, the median overall survival (OS) was more than 5 years, which was approximately one year longer than a comparator arm of hormone therapy alone. Although the combination of CDK4/6 inhibitors and endocrine therapy, an all-oral treatment that is generally well tolerated, had previously reported an OS benefit in premenopausal women with advanced HR+/HER2- breast cancer, this is the only first-line treatment to show an OS advantage in the postmenopausal population. MONALEESA-2 defines a new standard of care for this population.
Background
Despite advances that have increased the cure rate of early breast cancer, long-term survival has been elusive in advanced and metastatic disease. As recently as 4 years ago, fewer than 30% of women with metastatic breast cancer were still alive 5 years after their diagnosis.1 In advanced HR+/HER2- breast cancer, the introduction of endocrine therapies, such as tamoxifen, which inhibits activation of estrogen receptors,2 and aromatase inhibitors, which lower circulating levels of estrogen,3 have slowed disease progression substantially, but long-term survival has been uncommon.4 When combined with endocrine therapies, CDK4/6 inhibitors have provided a major additional step forward in disease control.5
HR+/HER2- disease is among the most common forms of breast cancer. Approximately 75% of all breast cancers are HR+, defined by expression of estrogen receptors, progesterone receptors, or both.6 According to an analysis of the U.S Surveillance, Epidemiology and End Results (SEER) registry in 2014, most are HER2-, defined by low levels of the HER2 protein assessed with histoscore or fluorescence in situ hybridization (FISH).7 Only 4.6% were HR- and HER2+. Of the remaining, 12.2% were triple negative, and HR/HER2 status was unknown in 12%. The hormone therapies tamoxifen, aromatase inhibitors, and fulvestrant modify the action of estrogen.8 They have had a major impact on improving outcomes in HR+ cancers,9 but median response duration in metastatic disease has remained limited, and the risk of endocrine resistance is substantial.10
CDK4/6 inhibitors, which interrupt intracellular and mitogenic hormone signals that stimulate proliferation of malignant cells,5 have proven highly effective in both first- and second-line treatment of HR+ breast cancer in numerous trials.11 CDK4/6 inhibitors target dysregulation of the cyclin D1-CDK4/6-Rb signaling cascade, which is an important mechanism of breast cancer cell proliferation.12 While resistance can develop to both endocrine therapies and to CDK4/6 inhibitors, the combination of these two classes of therapy appears to act synergistically to preserve efficacy of both.13
Palbociclib was the first CDK4/6 inhibitor approved by the US Food and Drug Administration in 2015. In Canada, there are now 3 oral CDK4/6 inhibitors licensed for use. Both ribociclib and palbociclib are indicated in combination with an endocrine therapy for first-line and second-line treatment of advanced or metastatic HR+/HER2- breast cancer. Abemaciclib has the same indications but is also approved as a single agent in patients with disease progression following endocrine therapy and at least 2 prior chemotherapy regimens.
Results and Implications of the MONALEESA-2 Trial
Ribociclib was granted an indication for first-line therapy in HR+/HER2- advanced or metastatic breast cancer on the basis of a large improvement in progression-free survival (PFS) in the phase 3 MONALEESA-2 trial.14 In that trial, 668 patients were randomized to ribociclib plus letrozole or letrozole plus placebo. The PFS in the control arm was 14.7 months but was not reached in the experimental arm at the time results were published. On the basis of the hazard ratio (HR), this translated into a 44% reduction in risk of progression for the combination versus letrozole plus placebo (HR 0.56; P=3.29 x 10-6). Ribociclib was well tolerated; grade 3 or higher adverse events were limited to cytopenias.
CDK4/6 inhibitors were evaluated in the first-line treatment of advanced or metastatic HR+HER-2 negative postmenopausal breast cancer in the PALOMA-2 trial and the MONARCH-3 trials.
In PALOMA-2, palbociclib plus letrozole was compared to letrozole plus placebo.15 By investigator assessment, palbociclib reduced the risk of progression by 35% (HR 0.65; P<0.001). In the MONARCH-3 trial, abemaciclib plus either of two aromatase inhibitors (anastrozole or letrozole) was compared to these endocrine therapies plus placebo.16 The advantage for PFS in the MONARCH-3 trial favoring abemaciclib was 46% (HR 0.54; P=0.000021).
As in MONALEESA-2, the CDK4/6 inhibitors in combination with endocrine therapy were generally well tolerated, and both trials led to regulatory approval for these indications.
Since completion of these trials, all three CDK4/6 inhibitors are now widely used for first-line therapy in advanced or metastatic HR+/HER2- breast cancer in postmenopausal women. OS was a predefined secondary study endpoint in all 3 trials, but the MONALEESA-2 trial was the only one of 3 trials that designated OS as a key secondary endpoint in its original design. Despite comparable follow-up with PALOMA-2 and only slightly shorter follow-up with MONARCH-3, MONALEESA-2 is the only one to demonstrate and OS advantage in follow-up to date.
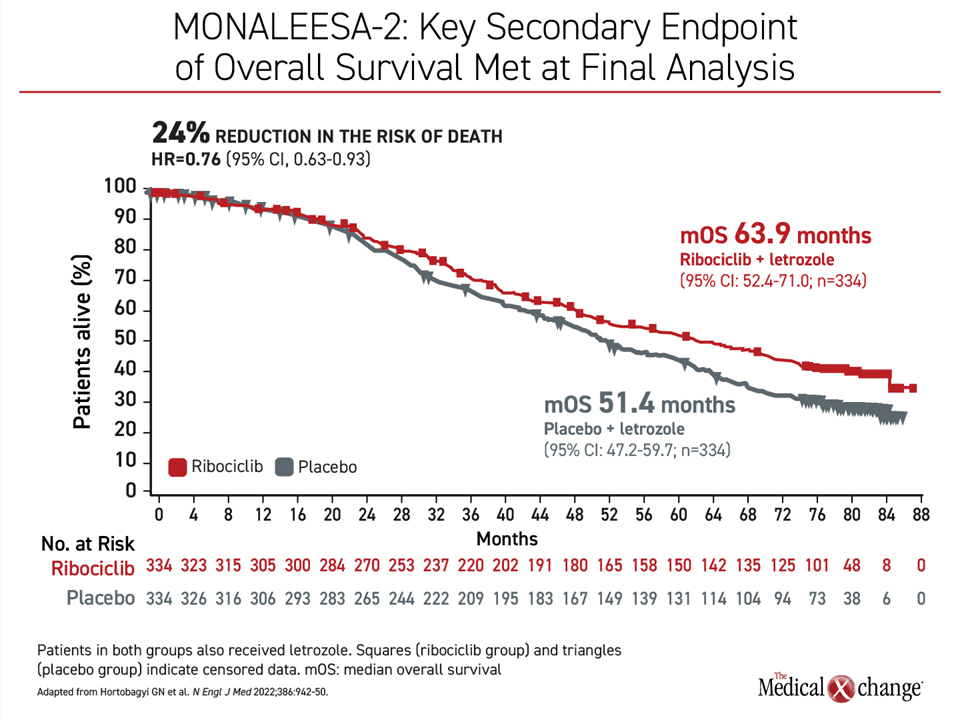
The OS benefit, which is typically employed to define a standard of care in oncology, was achieved despite subsequent second-line therapies. After 5 years of follow-up, 52.3% of those started on ribociclib and letrozole as first-line therapy versus 43.9% of those started on letrozole alone were still alive at 5 years. The median OS in the arm with ribociclib was 63.9 months or slightly more than 1 year greater than the 51.4-month median survival on letrozole alone. This translated to a 24% reduction (HR 0.765; P=0.004) in the risk of death in favor of the addition of ribociclib (Figure 1).
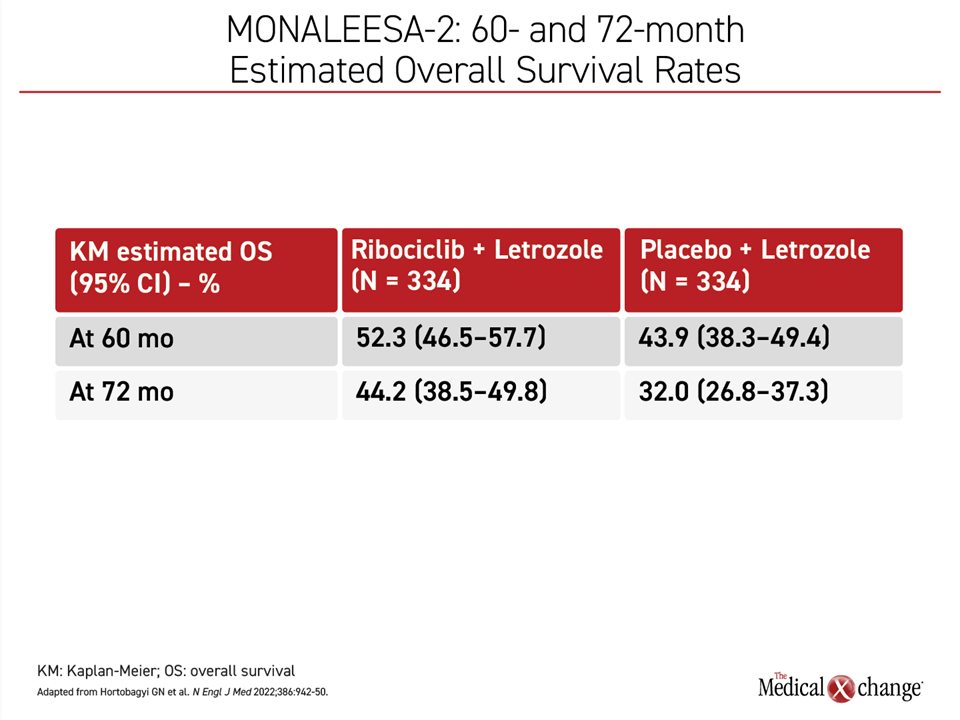
In MONALEESA-2, the survival curves started to separate at around 2 years with an increasing gap in favor of ribociclib plus letrozole over time, climbing from 17% relative advantage at the 60-month analysis to a 44% relative advantage (44.2% vs. 32.0%) after 72 months of follow-up (Figure 2).
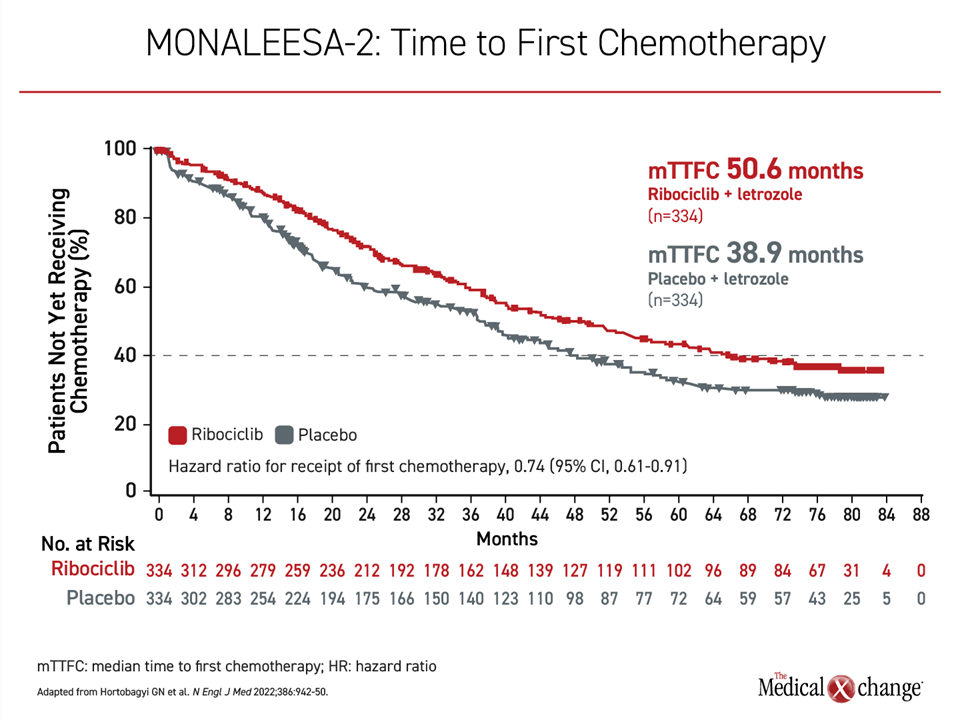
The extended survival was accompanied by prolonged disease control. Most importantly, the median chemotherapy-free survival was 39.9 months for those receiving the combination versus 30.1 months for those started on letrozole alone, which corresponds to a 25% reduction in time to chemotherapy (HR 0.742). Analyzed differently, the median time to first chemotherapy was more than 4 years in those randomized to ribociclib plus letrozole (50.6 months) to just over 3 years (38.9 months) in the group receiving letrozole alone (Figure 3).
OS benefits were consistent when patients were stratified by number of sites of metastases, progesterone receptor positivity, or prior versus no prior endocrine therapy.
With the large OS benefit associated with ribociclib plus letrozole over endocrine therapy alone in advanced or metastatic HR+/HER2- breast cancer in postmenopausal women, the MONALEESA-2 trial has set a new benchmark for survival in this population. The tolerability of ribociclib and the more than 1-year delay in the time to second-line therapies have major implications for preserving a good quality of life during the extended survival.
The MONALEESA-2 OS benefit of the ribociclib/endocrine therapy combination in postmenopausal women with previously untreated HR+/HER2- advanced breast cancer is consistent with the previously reported OS benefit in MONALEESA-3.17 This phase 3 trial enrolled postmenopausal women with HR+/HER2 breast cancer who had relapsed at least 12 months after initiating adjuvant or neoadjuvant endocrine therapy or who had received no more than one line of endocrine therapy in the advance setting. After a median 39 months of follow up, the median survival was 53.7 months among those randomized to ribociclib plus fulvestrant versus 41.5 months among those receiving fulvestrant alone, a risk reduction of 28% (HR 0.7255; P=0.00455).
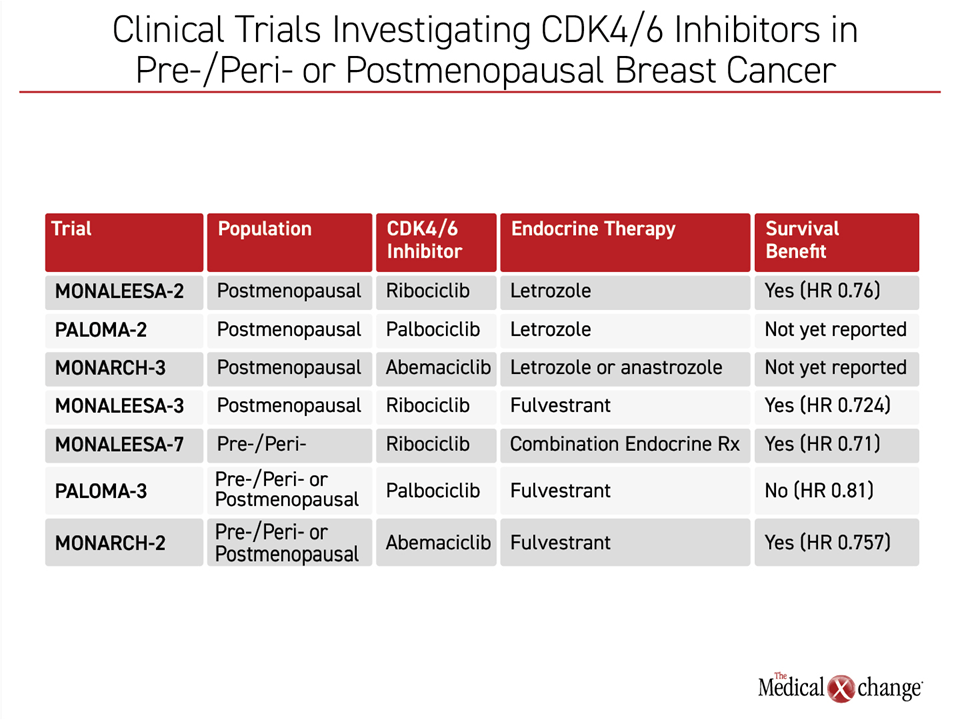
CDK4/6 Inhibitors in Pre-, or Peri-, or Postmenopausal Breast Cancer
The value of combining CDK4/6 inhibitors plus endocrine therapy has also been demonstrated in premenopausal women with HR+/HER2- breast cancer and studies that enrolled women without regard to menopausal status, including pre-, peri-, and post-menopausal women. MONALEESA-7 was the only trial in which postmenopausal status was an exclusion criterion, but 17% of women in MONARCH-2 were pre- or peri-menopausal as were 21% of the women in PALOMA-3. In each of the trials, the CDK4/6 inhibitor combined with endocrine therapy was compared to the same endocrine therapy plus placebo. Although cross trial comparisons are problematic due to potential differences in design or recruitment, the results of these trials are sufficiently different to raise the possibility that these CDK4/6 inhibitors are not interchangeable.
In the MONALEESA-7 trial, 672 patients were randomized to the CDK4/6 inhibitor ribociclib or placebo. Patients in both arms also took goserelin and either a nonsteroidal aromatase inhibitor or tamoxifen.17 In the PALOMA-3 trial, 525 patients were randomized in a 1:1 ratio.18 In the MONARCH-2 trial, 669 patients were randomized in a 2:1 ratio.19
An OS benefit was observed for the addition of the CDK4/6 inhibitor to endocrine therapy in MONALEESA-7 and MONARCH-2 but not in PALOMA-3.
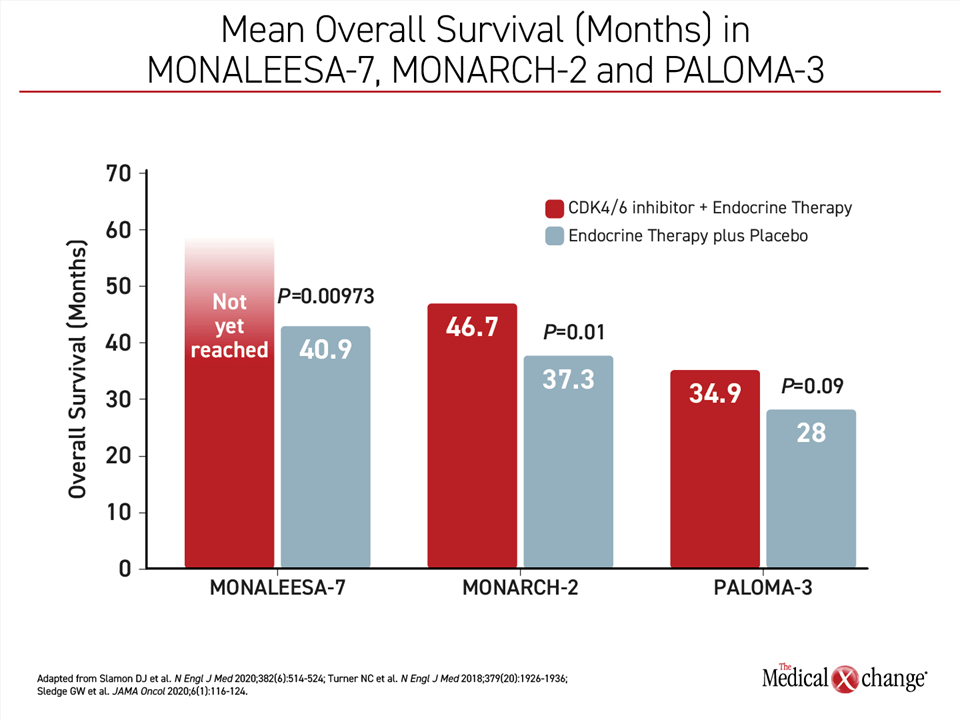
In MONALEESA-7, the estimated OS after a median 42 months of follow-up was 70.2% in the ribociclib group versus 46.0% in the placebo group, which translated into a 29% reduction in the risk of death (HR 0.71; P=0.000973). The median OS was 40.9 months in the placebo group but was not yet reached in the group randomized to ribociclib. The survival curves in MONALEESA-7 began to separate after about 28 months of follow-up and were continuing to widen at the time the study was published.
In the MONARCH-2 trial, the median OS was 46.7 months in the group receiving abemaciclib plus fulvestrant versus 37.3 months in the group receiving fulvestrant alone (P=0.01). As in MONALEESA-7, the benefit of the CDK4/6 inhibitor was consistent across all predefined stratification factors.
In PALOMA-3, the median OS was 34.9 months in the group receiving palbociclib plus fulvestrant versus 28.0 months in the group receiving fulvestrant alone. The hazard ratio of 0.81 in favor of palbociclib did not reach statistical significance (P=0.09) (Figure 4).
Although the design of the studies is similar, there were differences in the study populations other than the fact that postmenopausal women were included in MONARCH-2 and PALOMA-3. In the PALOMA 3 trial, for example, one prior chemotherapy in the advanced setting was allowed along with any number of prior endocrine therapies, resulting in a more heavily pretreated population. Thus, although MONARCH-2 and PALOMA-3 employed the same endocrine therapy comparator, the reasons for the differences in outcomes are only speculative. They may be related to study populations or differences in these drugs. Overall, there has been a similar direction favoring CDK4/6 inhibitors against the previous standard in HR+/HER2- breast cancer in younger and older women, but, again the absolute benefits across trials are not the same (Table 1).
It is clear that the combination of CDK4/6 inhibitors and endocrine therapy are highly active against HR+/HER2- advanced breast cancer whether women are or are not postmenopausal. In PALOMA-2, for example, there was a nearly 10-month delay in the time to first chemotherapy (17.6 vs. 8.8 months; P<0.001) for palbociclib versus endocrine therapy alone despite the absence of a significant OS benefit. This relative delay in progression, which was even greater in MONALEESA-7 and MONARCH-2, has implications for patient well-being and quality of life. Relative to the all-oral combination of a CDK4/6 inhibitor and endocrine therapy, second-line therapies, many of which require intravenous administration and have a high burden of adverse events, signal progression, making delay a meaningful goal in the effort to transform advanced breast cancer into a controllable disease.
Summary
The important message from the newly-published MONALEESA-2 trial in postmenopausal women with HR+/HER2- advanced and metastatic survival is that median survival of a progressive and ultimately fatal disease now exceeds 5 years when ribociclib and letrozole are initiated first-line. The oral combination therapy that provides this extended survival is simple and well tolerated. Most patients started on ribociclib and letrozole will not require first chemotherapy on average for more than 4 years. A substantial proportion of patients achieve even longer periods of disease-free survival suggests there is progress toward achieving indefinite disease control. This benefit is important for persons with advanced hormone positive, HER2 negative breast cancer.
References
1. El Sayed R, El Jamal L, El Iskandarani S, Kort J, Abdel Salam M, Assi H. Endocrine and Targeted Therapy for Hormone-Receptor-Positive, HER2-Negative Advanced Breast Cancer: Insights to Sequencing Treatment and Overcoming Resistance Based on Clinical Trials. Front Oncol 2019;9:510. DOI: 10.3389/fonc.2019.00510.
2. Yao J, Deng K, Huang J, Zeng R, Zuo J. Progress in the Understanding of the Mechanism of Tamoxifen Resistance in Breast Cancer. Front Pharmacol 2020;11:592912. DOI: 10.3389/fphar.2020.592912.
3. Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med 2003;348(24):2431-42. DOI: 10.1056/NEJMra023246.
4. Kaklamani VG, Gradishar WJ. Endocrine Therapy in the Current Management of Postmenopausal Estrogen Receptor-Positive Metastatic Breast Cancer. Oncologist 2017;22(5):507-517. DOI: 10.1634/theoncologist.2015-0464.
5. Scott SC, Lee SS, Abraham J. Mechanisms of therapeutic CDK4/6 inhibition in breast cancer. Semin Oncol 2017;44(6):385-394. DOI: 10.1053/j.seminoncol.2018.01.006.
6. Kohler BA, Sherman RL, Howlader N, et al. Annual Report to the Nation on the Status of Cancer, 1975-2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst 2015;107(6):djv048. DOI: 10.1093/jnci/djv048.
7. Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106(5). DOI: 10.1093/jnci/dju055.
8. Rasha F, Sharma M, Pruitt K. Mechanisms of endocrine therapy resistance in breast cancer. Mol Cell Endocrinol 2021;532:111322. DOI: 10.1016/j.mce.2021.111322.
9. Muss HB. Endocrine therapy for advanced breast cancer: a review. Breast Cancer Res Treat 1992;21(1):15-26. DOI: 10.1007/BF01811960.
10. Nicholson RI, Johnston SR. Endocrine therapy–current benefits and limitations. Breast Cancer Res Treat 2005;93 Suppl 1:S3-10. DOI: 10.1007/s10549-005-9036-4.
11. Spring LM, Wander SA, Zangardi M, Bardia A. CDK 4/6 Inhibitors in Breast Cancer: Current Controversies and Future Directions. Curr Oncol Rep 2019;21(3):25. DOI: 10.1007/s11912-019-0769-3.
12. An HX, Beckmann MW, Reifenberger G, Bender HG, Niederacher D. Gene amplification and overexpression of CDK4 in sporadic breast carcinomas is associated with high tumor cell proliferation. Am J Pathol 1999;154(1):113-8. DOI: 10.1016/S0002-9440(10)65257-1.
13. Lei JT, Anurag M, Haricharan S, Gou X, Ellis MJ. Endocrine therapy resistance: new insights. Breast 2019;48 Suppl 1:S26-S30. DOI: 10.1016/S0960-9776(19)31118-X.
14. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med 2016;375(18):1738-1748. DOI: 10.1056/NEJMoa1609709.
15. Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375(20):1925-1936. DOI: 10.1056/NEJMoa1607303.
16. Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol 2017;35(32):3638-3646. DOI: 10.1200/JCO.2017.75.6155.
17. Slamon DJ, Neven P, Chia S, et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N Engl J Med 2020;382(6):514-524. DOI: 10.1056/NEJMoa1911149.
18. Turner NC, Slamon DJ, Ro J, et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med 2018;379(20):1926-1936. DOI: 10.1056/NEJMoa1810527.
19. Sledge GW, Jr., Toi M, Neven P, et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol 2020;6(1):116-124. DOI: 10.1001/jamaoncol.2019.4782.