Cardiology
Heart Failure: Expert Review and Commentary from Published Literature
Guideline-Directed Therapies in Heart Failure for Optimal Survival: A Systematic Analysis
Dr. Lisa Mielniczuk, FRCPC
Professor of Medicine (Cardiology) / Cellular and Molecular Medicine
Tier 1 University of Ottawa Heart Function Research Chair
Vice-Chair Quality and Clinical Care, Department of Medicine
Director, Advanced Heart Failure Program, University of Ottawa Heart Institute
Ottawa, Ontario
Dr. Véronique Cyr, FRCPC
Cardiologist, Department of Medicine, Centre hospitalier de l’Université de Montréal (CHUM)
Assistant Professor of Medicine, University of Montreal
Montreal, Quebec
Each of the four pillars of guideline-recommended medications for heart failure with reduced ejection fraction (HFrEF) is associated with a mortality benefit independent of the other three. When a systematic analysis was recently conducted using data to compare the aggregate benefit of these therapies, the angiotensin neprilysin inhibitor (ARNI) led the list in the relative reduction in all-cause mortality. This is noteworthy. Of the four guideline-directed medical therapies (GDMT)—a beta blocker (BB), a mineralocorticoid receptor antagonist (MRA), an ARNI, and a sodium glucose co-transporter-2 inhibitor (SGLT2i)—the ARNI has been among the most commonly omitted, according to practice surveys. For the goal of improving survival in HFrEF patients, this is clinically meaningful. The systematic analysis confirmed a substantially greater mortality benefit for the ARNI sacubitril/valsartan relative to either angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB), which the ARNI replaced in current guidelines. It is important to recognize that the gain in mortality benefit with each of the four GDMT therapies is additive. The aggregate survival benefit for patients maintained on all four GDMT therapies is measured in years. Failure to initiate all four treatments promptly risks avoidable deaths.
Background
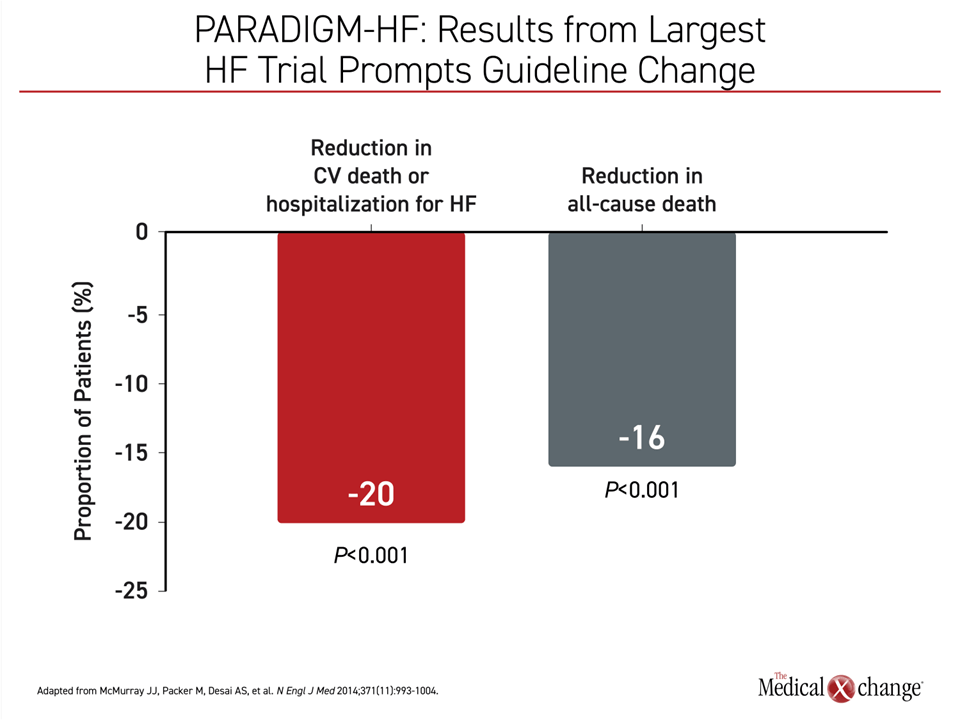
In 2017, treatment guidelines in Canada were updated to add an ARNI as a pillar of HFrEF treatment, replacing the renin-angiotensin system (RAS) inhibitors, whether an ACEi or an ARB.1 Guidelines issued in the same year by the American College of Cardiology, the American Heart Association, and the Heart Failure Society of America (ACC/AHA/HFSA) made the same change, creating a three-drug HFrEF standard consisting of BB, MRA, and the ARNI sacubitril/valsartan.2 In PARADIGM-HF, the multinational trial that led to the guideline change, sacubitril/valsartan provided a 16% reduction (HR 0.84; P<0.001) in death from any cause relative to the ACEi enalapril3 (Figure 1).
For an exclusive interview with Dr. Lisa Mielniczuk on the impact to clinical practice, click here
In 2021, treatment recommendations for HFrEF in Canada were updated again.4 These added a fourth agent, a SGLT2i, to GDMT. Again, like BB, MRA and ARNI, the change was based on a multinational trial, DAPA-HF.5 In this placebo-controlled trial, the SGLT2i dapagliflozin also achieved a significant reduction in all-cause mortality (HR 0.83; P value not calculated). This mortality benefit, like the mortality benefit associated with sacubitril/valsartan, was independent and additive to the previous HFrEF standard.
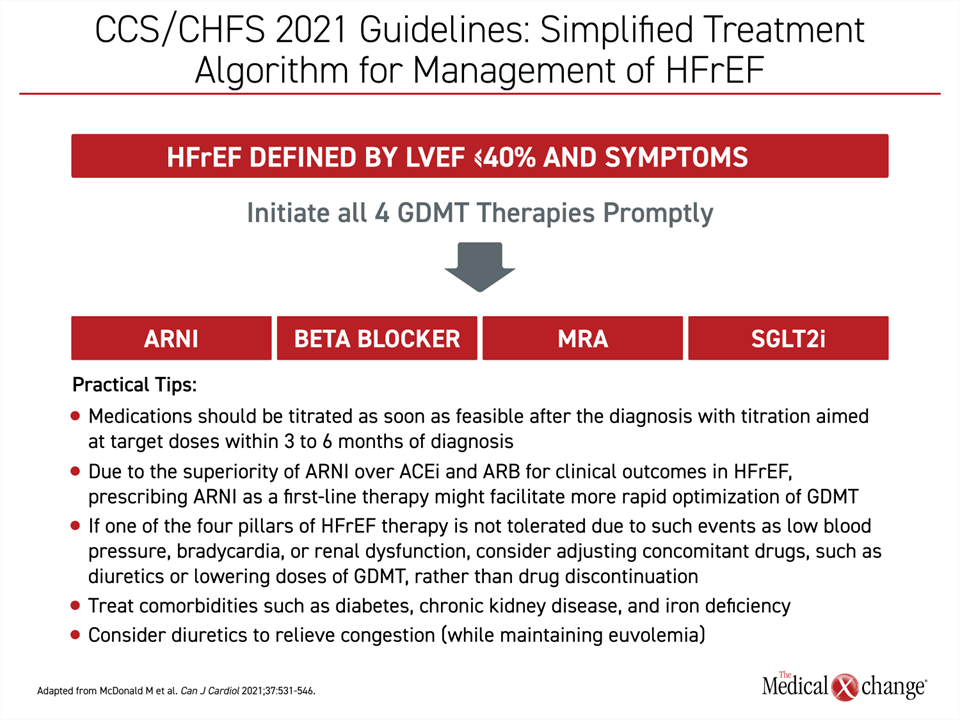
The 2021 CCS/CHFS updated guidelines included detailed strategies on how to start and integrate the four pillars of GDMT in patients with HFrEF, emphasizing the goal of introducing and uptitrating all four therapies as soon as feasible and no more than 6 months after diagnosis (Figure 2). While the earlier 2017 CCS/CHFS guidelines included a stepped approach for starting patients on an ACEi or ARB and switching to the ARNI, the 2021 guidelines identified ARNI as a first-line therapy that should be introduced promptly in symptomatic patients. This includes initiating sacubitril/valsartan at the time of hospitalization in a new diagnosis of HFrEF. These recommendations were largely echoed in a 2021 ACC update of HFrEF decision pathway, which also emphasized the importance of rapidly placing patients on the 4-drug GDMT HFrEF standard.6
ARNI in Heart Failure
The ARNI sacubitril/valsartan has a dual mechanism of action that explains its relative advantage over agents such as ACEi, that target the RAS alone. The value of inhibiting RAS activation, which contributes to cardiovascular (CV) impairment and HFrEF progression through several mechanisms, such as hypertension,7 has been demonstrated in numerous large trials, including the landmark SAVE study.8 However, it is now clear that HFrEF is accompanied by other types of neurohormonal overactivation that have led to the current multidrug standard of care.
In addition to the sympathetic nervous system, which is inhibited by BBs, there is now clear evidence that alterations in natriuretic peptides, particularly N-terminal pro-B type natriuretic peptide (NT-proBNP), are a targetable component of HFrEF pathophysiology.9 By inhibiting neprilysin, which degrades NT-proBNP as well as several other vasoactive peptides, such as bradykinin, the sacubitril component of sacubitril/valsartan preserves these peptides to counter the pathologic effects of vasoconstriction, sodium retention, and other factors contributing to ventricular remodeling.10,11 As a result, the neprilysin inhibitor sacubitril, when combined with the ARB valsartan, provides a more comprehensive benefit on the multifaceted components of neurohormonal overactivation. This was shown clinically with the mortality benefit in the PARADIGM-HF trial,3 but there is also substantial experimental and clinical evidence that neprilysin inhibition attenuates adverse cardiac remodeling to prevent adverse changes in CV structure, which is characteristic of HFrEF progression.12
When sacubitril/valsartan was introduced in 2017 by major guidelines as a standard of care in HFrEF, limited experience with this agent resulted in a slow uptake by clinicians. In the United States, a HFrEF registry indicated that less than 20% of patients were receiving target doses of the ARNI a year after guidelines were implemented.13 In Canada, a similar analysis conducted in the same year suggested that only 12% of eligible HFrEF patients were receiving sacubitril/valsartan.14 Based on the established mortality benefit of this therapy, it was estimated that nearly 3000 preventable deaths might have occurred in the previous year due to this omission.
The 2021 CCS/CHFS guidelines and the ACC 2021 decision pathway update have addressed the potential obstacles to full and routine implementation of GDMT in HFrEF. With suggested strategies for uptitration of each of the four recommended treatments, these revised guidelines placed particular emphasis on how and when to introduce the ARNI. Both sets of guidelines emphasized that the ARNI is a first-line therapy, eliminating the need for initial treatment with a RAS inhibitor. In the CCS/CHFS update, the PIONEER-HF trial was cited in support of initiating ARNI at first HFrEF hospital admission. In PIONEER-HF and its open-label extension, this strategy relative to a delayed start was associated with a lower rate of CV mortality and heart failure admissions.15,16
Systematic Review: Aggregate Benefit of GDMT
By clarifying and, in some cases, simplifying pathways to optimal pharmacologic management of HFrEF, the updated guidelines attempted to remove barriers. While emphasizing the importance of initiating all four pillars of GDMT, the guidelines acknowledge the value of integrating these standard therapies with other treatments, such as diuretics, digoxin, and the combination of hydralazine-isosorbide dinitrate (H-ISDN), to relieve symptoms and improve outcomes. However, both the CCS/CHFS guidelines and the ACC decision pathway established GDMT as a foundation upon which other therapies are considered. While additional treatments can improve quality of life and contribute to improved outcomes, the four agents in GDMT are unique in demonstrating improved survival in most or all patients with HFrEF.
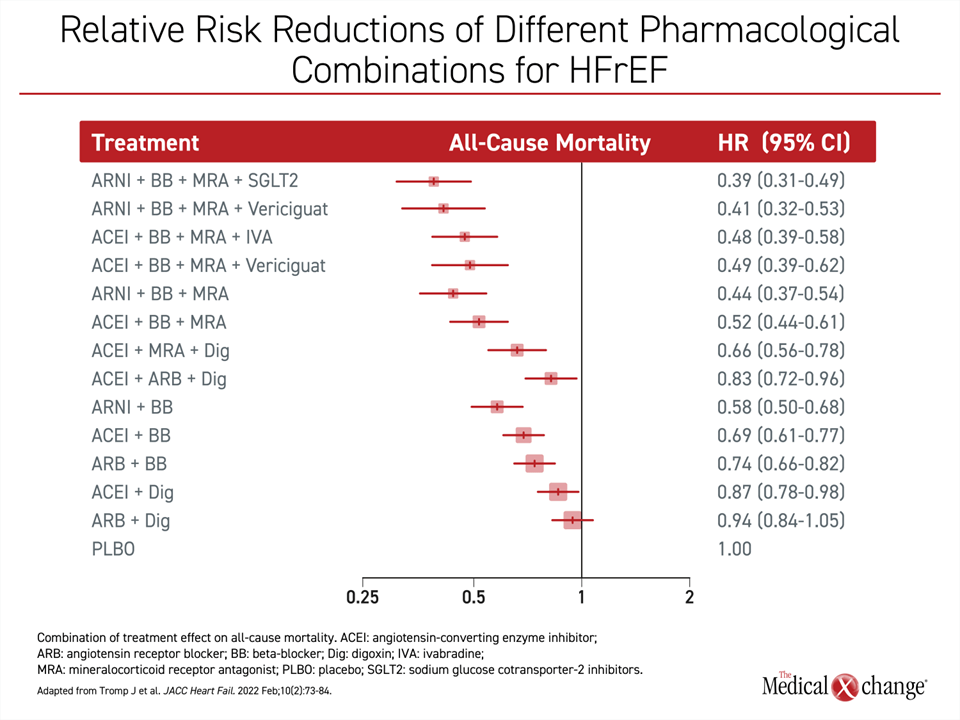
This is supported by a recently published systematic review and network meta-analysis of HFrEF treatments.17 In this analysis, the objective was to compare the aggregate treatment benefit of the current standard pharmacologic therapy for HFrEF. The data for this analysis was drawn from 75 randomized controlled trials that enrolled a total of 95,444 patients. The trials evaluated BB, MRA, the ARNI sacubitril/valsartan, SGLT2is, ACEis, ARBs, digoxin, H-ISDN, vericiguat, and omecamtiv-mecarbil.
The primary endpoint was all-cause death. The study evaluated the relative mortality impact on various combinations of these therapies and also employed an additive component network meta-analysis methodology to evaluate the contribution of individual components in the combination. In a secondary analysis, the absolute number of life-years gained with these combinations was estimated in two heart failure populations.
The meta-analysis provided powerful support for the guidelines. On the basis of all-cause mortality, the greatest reduction in the hazard ratio (HR) for mortality was, as specified in modern guidelines, the combination of an ARNI, BB, MRA, and SGLT2i. Relative to placebo, this was associated with a 61% reduction in risk of death (Figure 3). Other combinations were also associated with substantial mortality benefits. For example, the combination of ARNI, BB, MRA and vericiguat, a soluble guanylate cyclase stimulator which was associated with a reduction in CV mortality (but not all-cause mortality) in a phase III trial,18 provided a mortality reduction only slightly less than the GDMT standard. Other combinations were associated with all-cause mortality reductions of a lesser degree.
The order of relative benefit for the combinations remained the same for the endpoints of all-cause mortality and the composite of CV mortality or heart failure hospitalizations. In each case, the ARNI/BB/MRA/SGLT2i combination was associated with the greatest relative risk reduction.
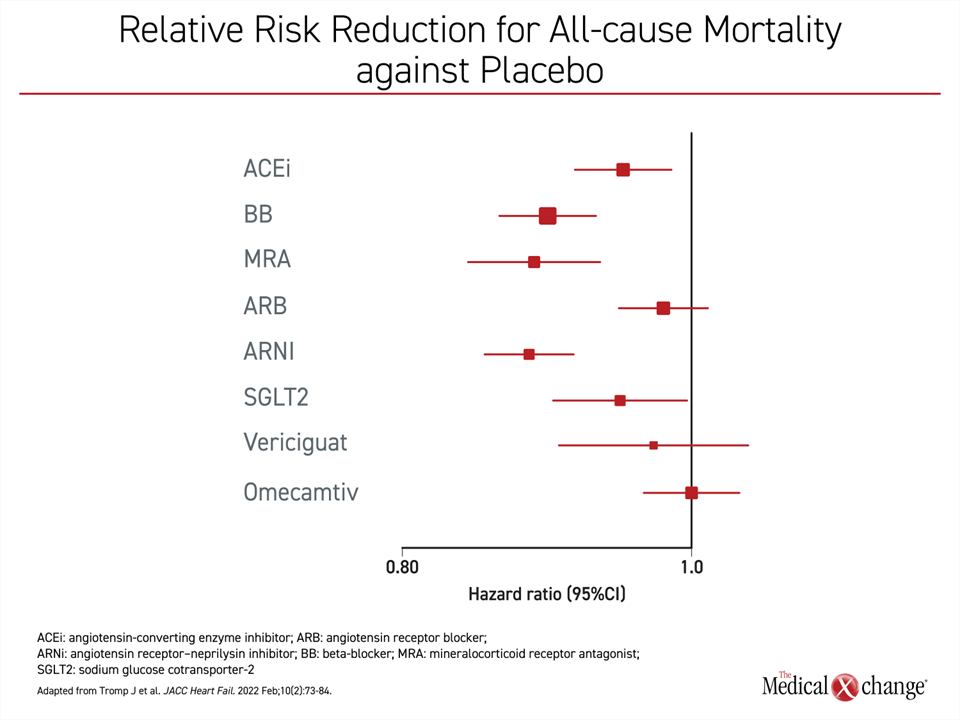
Among individual agents, the ARNI sacubitril/valsartan edged out MRA, providing a marginal but numerically superior greater all-cause mortality risk reduction relative to placebo (Figure 4). BB therapy followed but the difference was again marginal whether compared to MRA or ARNI. Of the four pillars of current GDMT, SGLT2i provided about half the mortality benefit of either ARNI or MRA even though differences did not reach statistical significance. In this analysis, the ACEi offered a protection against all-cause mortality that was also less than half of ARNI or BB. The all-cause mortality reduction of the ARB did not reach statistical significance.
In a secondary analysis, the estimated life-years gained from taking the GDMT of ARNI/BB/MRA/SGLT2i relative to placebo was 7.9 years for a 50-year-old with HFrEF. For a 70-year-old, the gain was an estimated 5.0 life years. When compared to actual care in comparative arms that included active HFrEF treatments, the gain was 4.9 and 3.3 years for those 50-years-old and 70-years-old, respectively.
This relative improvement in survival is consistent with a previous study that combined data from pivotal trials.19 In that study, using data from PARADIGM-HF with sacubitril-valsartan,3 EMPHASIS-HF with the MRA eplerenone,20 and DAPA-HF with the SGLT2i dapagliflozin,5 there was an estimated 6.3 year gain in a 55-year-old for the four-drug combination relative to BB and a RAS inhibitor.
Seizing the Opportunity of Early and Optimal Application of GDMT for HFrEF in Clinical Practice
For the clinician, there are several messages from newly published systematic analysis. The most important is that the mortality reductions are substantial for each of the GDMT agents, and they are additive. This is consistent with their effects on independent mechanisms of heart failure progression.
These data also emphasize that omission of one or more of these therapies or providing these therapies at less than optimal dosing will result in preventable early deaths. As each of these treatments inhibit compensatory but ultimately counterproductive pathways of neurohormonal activation and cardiovascular remodeling, a cumulative benefit is expected over time, encouraging prompt initiation of treatment in advance of structural damage.
The CCS/CHFS and other major guidelines emphasize the importance of striving to provide all four GDMT therapies at optimal doses. Of these therapies, the ARNI sacubitril/valsartan is not an exception. The systematic analysis identifies it as a critical contributor to improved survival whether considered for its individual contribution or in the context of an optimal combination.
GDMT for HFrEF as defined by the CCS/CHFS is evidence-based. The efforts to improve adherence to the four pillars of treatment is driven by the goal of improving survival. All four of the GDMT therapies are well tolerated and employ relatively simple oral dosing regimens. The major risks, such as hypotension with rapid titration of the combined therapy, can largely be avoided by the strategies recommended in the 2021 CCS/CHFS guideline update and the 2021 ACC decision pathway summary. In achieving the goal of placing all HFrEF patients on optimal therapy, these guidelines are relevant to specialists as well as clinicians in primary care, who provide routine care to a substantial proportion of HFrEF patients.
Summary
Over the past 30 years, the progress in recognizing targetable pathways of HFrEF progression have led to substantial incremental gains in survival. In a series of landmark trials, four therapies have now succeeded in improving survival when added to the previous treatment standard. Used together, these have a cumulative benefit of years of additional life. At the time that the ARNI sacubitril-valsartan was included as the third pillar of GDMT in HFrEF, its incorporation into routine practice was disappointingly slow. Now with the addition of SGLT2i, a fourth GDMT, there is renewed emphasis on conveying that the benefits of each therapy are additive, making omission of any agent a definition of suboptimal care and a lost opportunity for optimal survival benefits.
References
1. Ezekowitz JA, O’Meara E, McDonald MA, et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can J Cardiol 2017;33(11):1342-1433. DOI: 10.1016/j.cjca.2017.08.022.
2. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70(6):776-803. DOI: 10.1016/j.jacc.2017.04.025.
3. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371(11):993-1004. DOI: 10.1056/NEJMoa1409077.
4. McDonald M, Virani S, Chan M, Ducharme A. CCS/CHFS heart failure guidelines update: defining a new pharmacologic standard of care for heart failure with reduced ejection fraction. Can J Cardiol 2021;37:531-546.
5. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019;381(21):1995-2008. DOI: 10.1056/NEJMoa1911303.
6. Maddox TM, Januzzi JL, Jr., Allen LA, et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2021;77(6):772-810. DOI: 10.1016/j.jacc.2020.11.022.
7. Pfeffer MA, Lamas GA, Vaughan DE, Parisi AF, Braunwald E. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med 1988;319(2):80-6. DOI: 10.1056/NEJM198807143190204.
8. Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 1992;327(10):669-77. DOI: 10.1056/NEJM199209033271001.
9. Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol 2017;14(1):30-38. DOI: 10.1038/nrcardio.2016.163.
10. Fu S, Ping P, Zhu Q, Ye P, Luo L. Brain Natriuretic Peptide and Its Biochemical, Analytical, and Clinical Issues in Heart Failure: A Narrative Review. Front Physiol 2018;9:692. DOI: 10.3389/fphys.2018.00692.
11. Kuhn M. Molecular physiology of natriuretic peptide signalling. Basic Res Cardiol 2004;99(2):76-82. DOI: 10.1007/s00395-004-0460-0.
12. Januzzi JL, Jr., Prescott MF, Butler J, et al. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA 2019:1-11. DOI: 10.1001/jama.2019.12821.
13. Greene SJ, Butler J, Albert NM, et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol 2018;72(4):351-366. DOI: 10.1016/j.jacc.2018.04.070.
14. Huitema AA, Daoust A, Anderson K, et al. Optimal Usage of Sacubitril/Valsartan for the Treatment of Heart Failure: The Importance of Optimizing Heart Failure Care in Canada. CJC Open 2020;2(5):321-327. DOI: 10.1016/j.cjco.2020.03.015.
15. Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med 2019;380(6):539-548. DOI: 10.1056/NEJMoa1812851.
16. DeVore AD, Braunwald E, Morrow DA, et al. Initiation of Angiotensin-Neprilysin Inhibition After Acute Decompensated Heart Failure: Secondary Analysis of the Open-label Extension of the PIONEER-HF Trial. JAMA Cardiol 2020;5(2):202-207. DOI: 10.1001/jamacardio.2019.4665.
17. Tromp J, Ouwerkerk W, van Veldhuisen DJ, et al. A Systematic Review and Network Meta-Analysis of Pharmacological Treatment of Heart Failure With Reduced Ejection Fraction. JACC Heart Fail 2022;10(2):73-84. DOI: 10.1016/j.jchf.2021.09.004.
18. Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2020;382(20):1883-1893. DOI: 10.1056/NEJMoa1915928.
19. Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet 2020;396(10244):121-128. DOI: 10.1016/S0140-6736(20)30748-0.
20. Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364(1):11-21. DOI: 10.1056/NEJMoa1009492.