Oncology
Biliary Tract Cancer: Expert Review and Commentary from Published Literature
A Promise for Change in First-line Standard of Care for Biliary Tract Cancer
Vincent Tam, BSc(Hon), MD, FRCPC
Medical Oncologist, Tom Baker Cancer Centre
Clinical Associate Professor, Department of Oncology
Cumming School of Medicine, University of Calgary
Calgary, Alberta
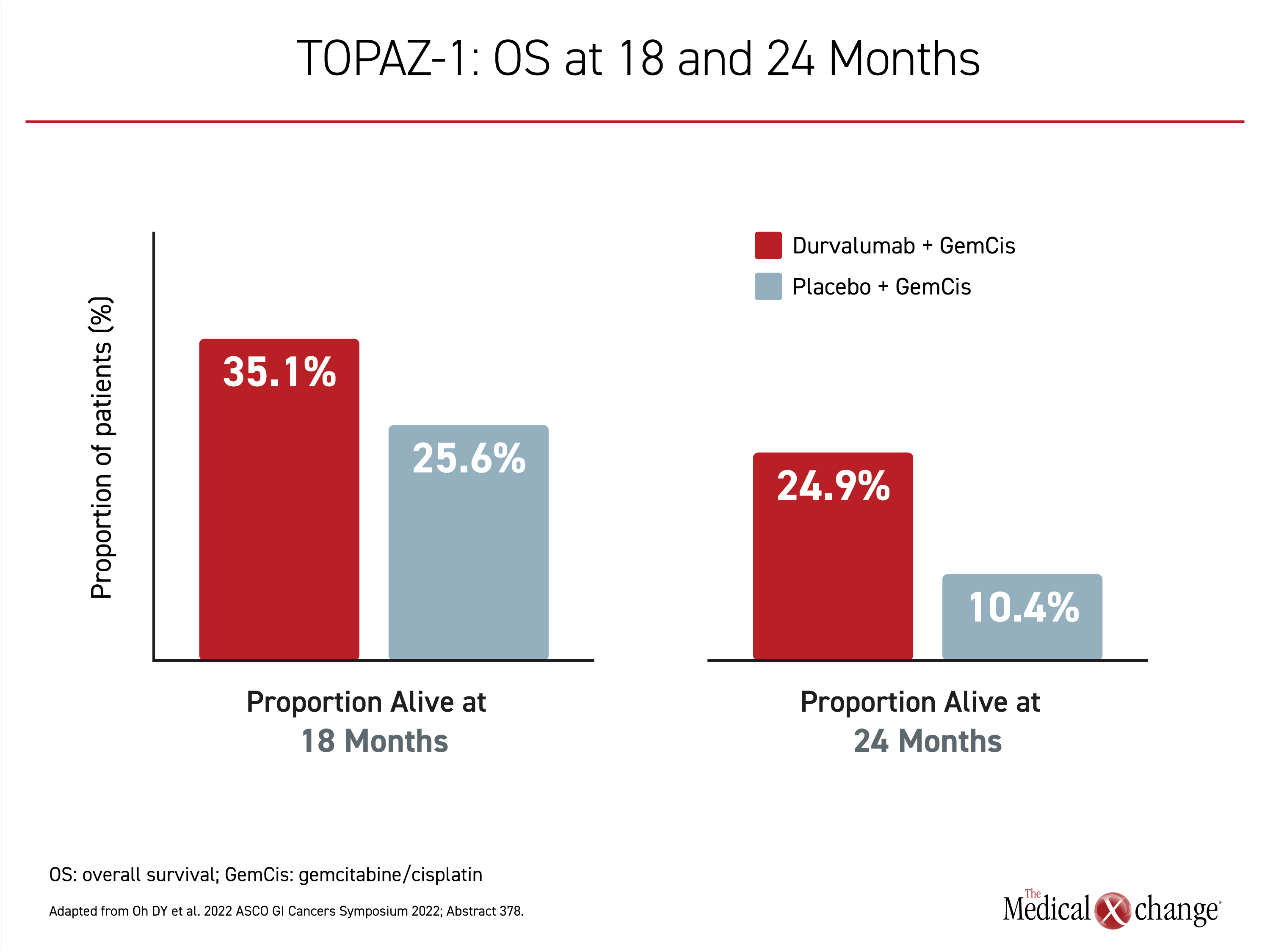
The first-line standard of care treatment of advanced, unresectable biliary tract cancer (BTC) is expected to change with the results of the recently completed TOPAZ-1 trial. In that phase 3 study, the combination of gemcitabine plus cisplatin (GemCis) chemotherapy was compared to the same therapy plus durvalumab, an immune checkpoint inhibitor (CPI). A statistically significant improvement in overall survival (OS) was shown with the addition of durvalumab, and the treatment was well tolerated. There were no differences in quality of life for those randomized to GemCis plus durvalumab relative to GemCis alone in a recent patient-reported outcomes (PRO) subanalysis. More than 10 years ago, when the addition of cisplatin to gemcitabine displaced single-agent gemcitabine as the first-line therapy in advanced BTC, the median OS increased by approximately 40%, reaching nearly 1 year. In the experimental arm of TOPAZ-1, there was a further 20% reduction in risk of death (HR 0.80; P=0.21) with a median OS now exceeding 1 year. The relative OS advantage increased over time and at 24 months, the proportion of survivors was more than 2-fold greater (24.9% vs. 10.4%).
Background
BTC, commonly categorized as intrahepatic, perihilar or extrahepatic in location, can arise in the epithelium anywhere along the biliary tract.1 Almost all BTC are cholangiocarcinomas, although other tumour types, such as sarcoma, do occur. BTC, and more specifically, cholangiocarcinoma is heterogeneous by genomic, epigenetic, and molecular characteristics.2 Rates of BTC vary globally, but cancers of this type occur at a low incidence overall in Western countries.3 In a Canadian survey, the estimate was about 4 cases per 100,000 individuals per year.4 These aggressive cancers are typically diagnosed at a late stage, making them a particularly lethal form of malignancy.
For an exclusive interview with Dr. Vincent Tam on the impact to clinical practice, click here
The etiology of BTC is likely to involve a complex interaction between genetic, lifestyle, and environmental triggers. Although parasitic infections, primary sclerosing cholangitis, and exposure to toxins, such as tobacco, are among sources of damage to the bile duct suspected of inducing malignant transformation,5 more than half of patients with BTC have no identifiable risk factors.6 Environmental factors are suspected of driving the geographic variability in the incidence and prevalence of BTC.
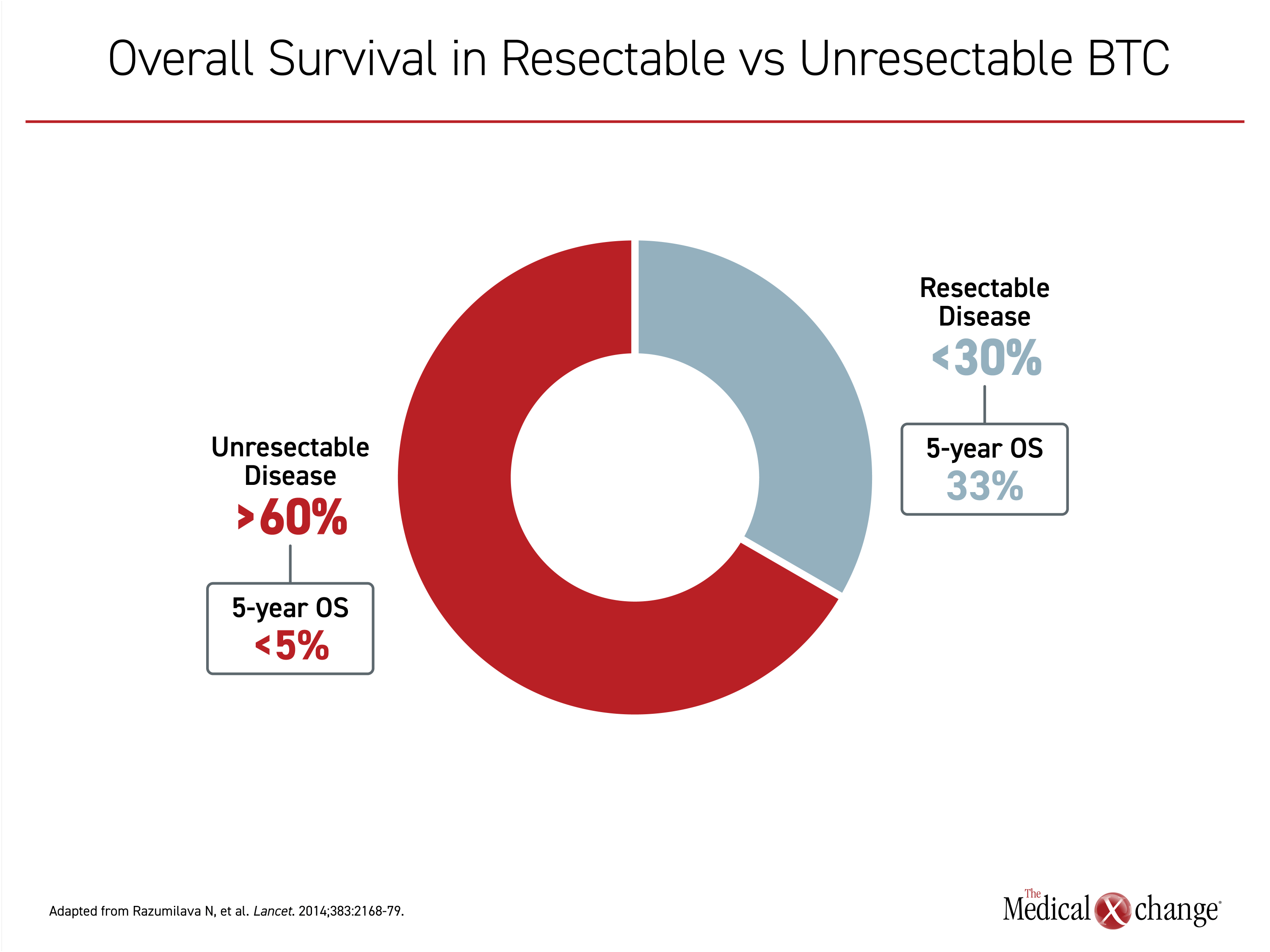
In patients with BTC, surgery is potentially curative,7 but more than 60% of patients have unresectable or metastatic disease at the time of diagnosis.1 Even when surgery is performed successfully, recurrence is common. In one study, the cure rate 9.5 years after surgery was less than 15%.8 In general, the 5-year OS rate is estimated at 33% for those with resectable disease but less than 5% for those with unresectable tumours (Figure 1).9 In the absence of systemic therapies, the estimated median OS of BTC patients managed with best supportive care is less than 6 months.10
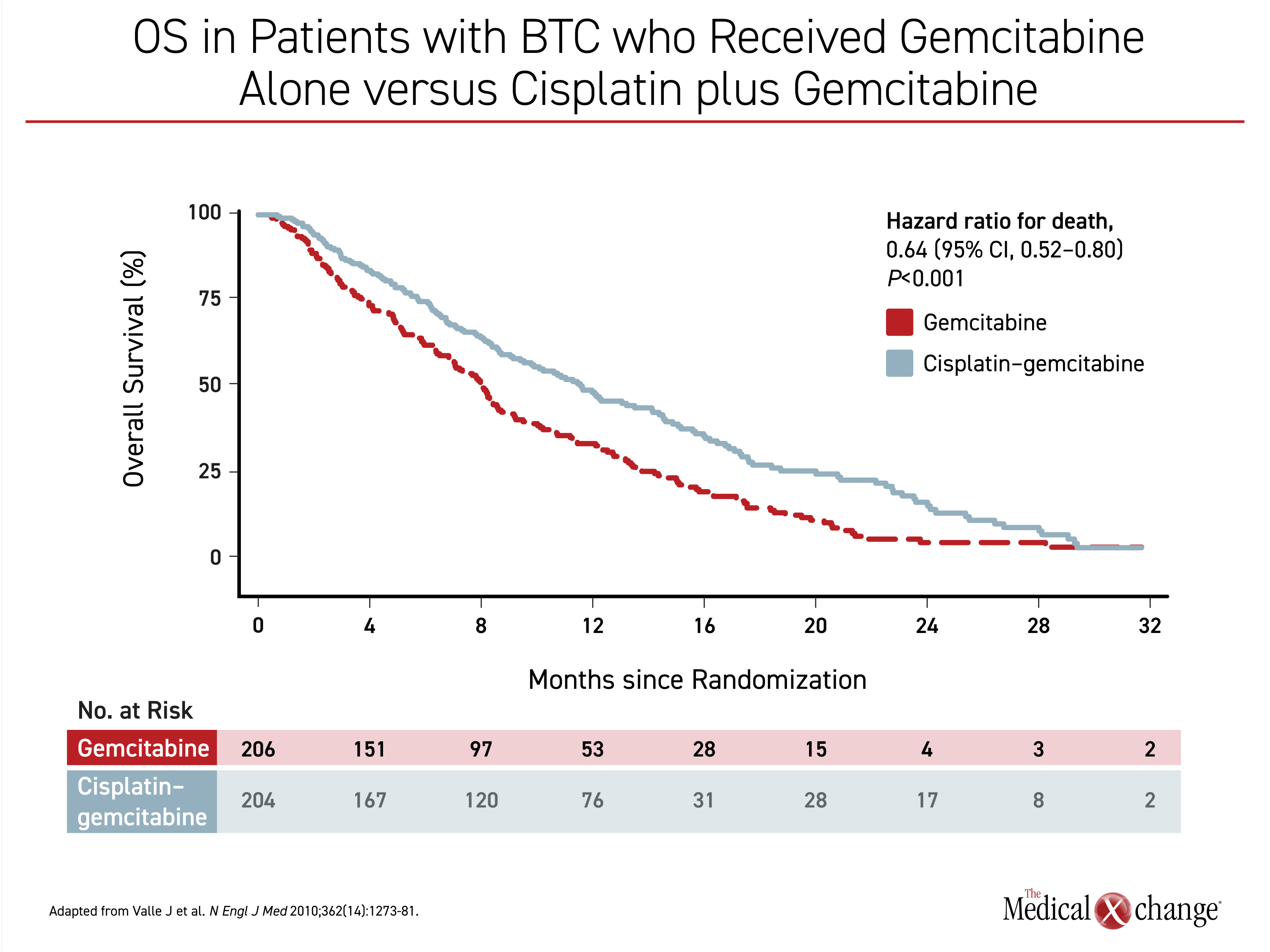
The first-line standard treatment for unresectable, recurrent, or metastatic BTC has been GemCis for more than 10 years. This standard was established by the ABC-02 trial which found that the addition of cisplatin to gemcitabine was associated with a highly significant reduction in the risk of death relative to gemcitabine alone (HR 0.64; P<0.001).11 In that trial, median OS improved from 8.1 months in the gemcitabine-alone arm to 11.7 months in the GemCis arm (Figure 2). The median progression-free survival (PFS) improved from 5.0 to 8.0 months (P<0.001). Neutropenia occurred more frequently with combination therapy, but neutropenia-associated infections did not. Adverse events were otherwise similar.
Evolving Role of Checkpoint Inhibitors in BTC
A study by Le et al enrolled patients with tumours expressing high microsatellite instability (MSI-H) and treated the patients with single-agent checkpoint inhibitor (CPI) therapy. This study included a small number of BTC cases.12 After 12.5 months of follow-up, median PFS and median OS had not yet been reached. Complete responses (CR) were achieved in a minority of patients, but a subset of these patients remained relapse free at the time of publication despite therapy discontinuation.
Studies of CPI therapy specifically in BTC followed. Most were conducted in patients who had relapsed after a prior treatment13,14 and many evaluated a single agent. Many of the studies enrolled patients with biomarkers potentially favourable for response to CPIs, such as MSI-H, a deficiency in mismatch repair (MMR), a high tumour mutation burden, or a high surface expression of the target checkpoint, such as PD-1. On the basis of ORR, which was often the primary endpoint, response to single agent CPIs was generally modest, but durable responses were seen in some patients.15,16
In the first-line BTC setting, studies have been more likely to evaluate CPI therapies in combination with other active agents, such as one or more cytotoxic agents or targeted therapies.14 BTC has characteristics that make immunotherapy attractive. For one, many patients with BTC express one or more of the biomarkers associated with CPI response, such as expression of the target checkpoint inhibitor, a high tumour mutation burden, or MMR deficiency.14 For another, relative intratumor immune activity, such as presence of tumour infiltrating lymphocytes, is a variable that appears to influence outcomes in BTC.17 While inflammatory activity is implicated in the etiology of BTC,18 response to CPI is likely linked to the ability of a suppressed immune response to malignancy to be revived. The presence of adaptive immune cells in the microenvironment of BTC increases the potential for clinically significant anti-tumour activity when checkpoint proteins are inhibited.
However, the role of factors that potentially influence response to CPI have not been well studied in clinical studies of BTC. Rather, most studies, including a phase 2 evaluation of CPI combination therapies led by Dr. Do-Youn Oh, Division of Medical Oncology, Seoul National University Hospital, Seoul, South Korea, and lead investigator of the subsequent phase 3 TOPAZ-1 trial,19 have not required biomarkers for enrollment.
In this phase 2 study, the initial design called for all patients to receive one cycle of GemCis. Starting in the second cycle, durvalumab alone or durvalumab plus the CTLA-1 checkpoint inhibitor tremelimumab were added. However, the protocol was subsequently amended so that patients received the checkpoint inhibitors along with chemotherapy on day 1 of the first cycle. Of the 128 treated patients, 32 were treated prior to the protocol amendment. Of the remaining, 49 received durvalumab plus chemotherapy starting in the first cycle and 47 received chemotherapy plus durvalumab and tremelimumab.
The ORR rate was 50% for patients treated prior to the change in protocol. For those treated after the protocol amendment, the ORR was 72% and 70% for the chemotherapy plus durvalumab and the chemotherapy plus durvalumab and tremelimumab, respectively. Both regimens were associated with acceptable tolerability. The most common grade ≥3 adverse events were hematologic, such as neutropenia (53%) and anemia (40%). Given the similar efficacy of the two arms, a phase 3 trial was planned with the single CPI addition of durvalumab.
TOPAZ-1 Study: Design and Outcomes
The multinational phase 3 TOPAZ-1 trial, which was first presented at the 2022 American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium, is the first multinational controlled study to evaluate a CPI as first-line therapy in BTC.20 In this study, durvalumab, an inhibitor of the PD-1 ligand (PD-L1), was compared to placebo when both were combined with the standard GemCis.
In TOPAZ-1, 685 patients untreated for unresectable locally advanced, recurrent, or metastatic BTC were randomized. In 3-week cycles, 1000 mg/m2 of gemcitabine plus 25 mg/m2 of cisplatin were administered on days 1 and 8 to all patients. Durvalumab or placebo was administered by infusion every 3 weeks. The GemCis combination chemotherapy was continued for up to 8 cycles followed by durvalumab or placebo alone until disease progression or unacceptable toxicity.
The primary endpoint was OS, with secondary endpoints including ORR, PFS, and safety. The median age was 64 years in both arms and about half were female. There was no significant difference in baseline ECOG performance status.
After a median follow-up of about 16 months, the median OS in the arm of GemCis with durvalumab was 12.8 months compared to 11.5 months in the GemCis alone arm. The advantage for the addition of the CPI translated into a 20% risk reduction in the OS endpoint (HR 0.80; P=0.021). The relative OS advantage for GemCis + durvalumab over chemotherapy alone was demonstrated, even with follow-up extended to 2 years (Figure 3). The median PFS improved from 5.7 months in the GemCis control arm to 7.2 months in the arm receiving GemCis with durvalumab, a risk reduction of 25% (HR 0.75; P=0.001). The ORRs were 26.7% and 18.7%, respectively.
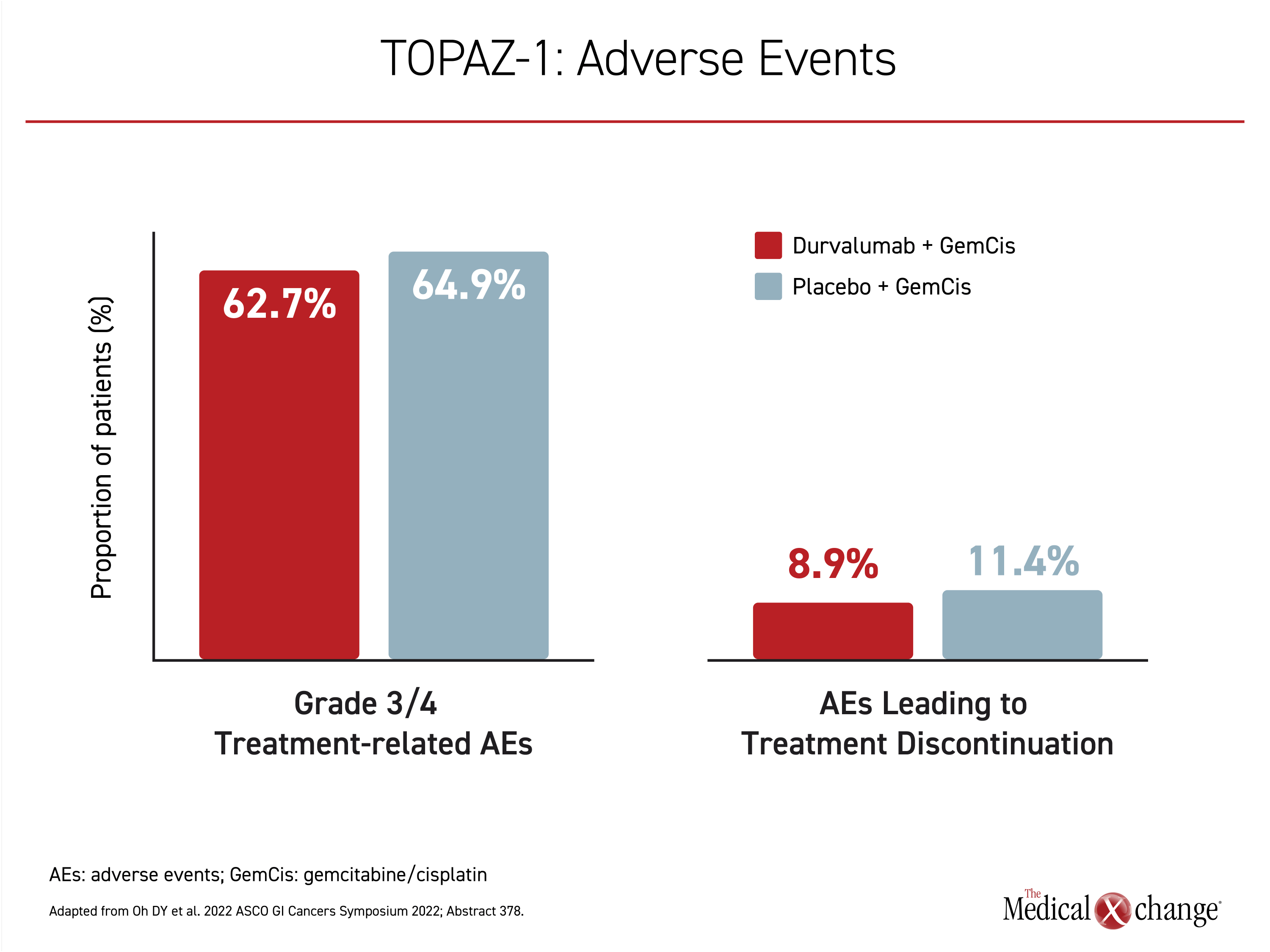
Grade 3 or 4 adverse events were observed in 62.7% of those randomized to the experimental arm and 64.9% of those receiving placebo plus chemotherapy. Most of these adverse events involved cytopenias or abnormal liver function tests. Of symptomatic grade ≥3 adverse events, fatigue was the most common, but was experienced by <20% of patients in either arm. Most other symptomatic adverse events, including nausea and alopecia, occurred in <10%. Discontinuation of treatment for adverse events occurred in 8.9% of patients receiving durvalumab plus chemotherapy and 11.4% of those receiving chemotherapy plus placebo (Figure 4).
In a pre-planned subanalysis of TOPAZ-1 presented at the 2022 ASCO Annual Meeting, results showed no significant difference in patient-reported QoL outcomes for treatment with GemCis combined with durvalumab compared to GemCis plus placebo. Time to deterioration (TTD) of quality of life, as assessed by EORTC QLQ-C30, showed a trend toward longer TTD in the durvalumab with GemCis arm.21 Evaluated with the previously validated EORTC QLQ-C30 and the TC 21-item module instruments, compliance in completing the forms in the first cycle (81%) remained high (>70%) at the end of the study of up to 28 cycles.
The OS benefit provided by durvalumab in the absence of any substantial increase in risk of adverse events provides the rationale for declaring GemCis with durvalumab a new standard of care. The quality-of-life analyses and tolerability profile in this randomized trial substantiate the premise that the extended survival is achieved with a similar and acceptably low risk of serious adverse events.
Summary
There is a strong unmet need for new options to treat unresectable BTC. The combination of GemCis has been a first-line treatment for over 10 years, but the median OS on this regimen is less than 1 year. With the addition of the CPI durvalumab to GemCis in the phase 3 TOPAZ-1 trial, median OS has now been pushed past 1 year with a larger proportion of patients still alive at 2 years. It is important to note that over the course of treatment, the addition of durvalumab to GemCis was well tolerated, and the increase in OS was achieved with little to no additional toxicity. Until additional treatment advances in BTC are made the absolute survival of these patients remains limited and maximizing survival while minimizing treatment-related toxicities and maintaining quality of life is a particularly meaningful goal.
References
1. Manne A, Woods E, Tsung A, Mittra A. Biliary Tract Cancers: Treatment Updates and Future Directions in the Era of Precision Medicine and Immuno-Oncology. Front Oncol 2021;11:768009. DOI: 10.3389/fonc.2021.768009.
2. Tariq NU, McNamara MG, Valle JW. Biliary tract cancers: current knowledge, clinical candidates and future challenges. Cancer Manag Res 2019;11:2623-2642. DOI: 10.2147/CMAR.S157092.
3. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17(9):557-588. DOI: 10.1038/s41575-020-0310-z.
4. Xiao Y, Cattelan L, Lagace F, et al. Epidemiologic trends and geographic distribution of patients with gallbladder and extrahepatic biliary tract cancers in Canada. HPB (Oxford) 2021;23(10):1541-1549. DOI: 10.1016/j.hpb.2021.03.007.
5. Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54(1):173-84. DOI: 10.1002/hep.24351.
6. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int 2019;39 Suppl 1:19-31. DOI: 10.1111/liv.14095.
7. Radtke A, Konigsrainer A. Surgical Therapy of Cholangiocarcinoma. Visc Med 2016;32(6):422-426. DOI: 10.1159/000452921.
8. Dhanasekaran R, Hemming AW, Zendejas I, et al. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol Rep 2013;29(4):1259-67. DOI: 10.3892/or.2013.2290.
9. Song W, Miao DL, Chen L. Survival rates are higher in married patients with biliary tract cancer: a population-based study. Oncotarget 2018;9(10):9531-9539. DOI: 10.18632/oncotarget.24170.
10. Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 2010;28(30):4581-6. DOI: 10.1200/JCO.2010.29.3605.
11. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362(14):1273-81. DOI: 10.1056/NEJMoa0908721.
12. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357(6349):409-413. DOI: 10.1126/science.aan6733.
13. Taghizadeh H, Prager GW. Immune Checkpoint Inhibitors for Advanced Biliary Tract Cancer. Curr Cancer Drug Targets 2022. DOI: 10.2174/1568009622666220215144235.
14. Jakubowski CD, Azad NS. Immune checkpoint inhibitor therapy in biliary tract cancer (cholangiocarcinoma). Chin Clin Oncol 2020;9(1):2. DOI: 10.21037/cco.2019.12.10.
15. Bang JH, Ueno M, Malka D, H.C. C. Pembrolizumab for advanced biliary adenocarcinoma: results from the KEYNOTE-028 (N+KN028) and KEYNOTE-158 (KN158) basket studies. American Society of Clinical Oncology Annual Meeting 2019;Abstract 4079.
16. Kim RD, Kim DW, Alese OB. A phase II study of nivolumab in patients with advanced refractory biliary tract cancers. American Society of Clinical Oncology Annual Meeting 2019;Abstract 4097.
17. Goeppert B, Frauenschuh L, Zucknick M, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer 2013;109(10):2665-74. DOI: 10.1038/bjc.2013.610.
18. Augustine MM, Fong Y. Epidemiology and risk factors of biliary tract and primary liver tumors. Surg Oncol Clin N Am 2014;23(2):171-88. DOI: 10.1016/j.soc.2013.10.001.
19. Oh DY, Lee KH, Lee DW, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol 2022;7(6):522-532. DOI: 10.1016/S2468-1253(22)00043-7.
20. Oh DY, He AR, Qin S, Chen L. A phase 3 randomized, double-blind, placebo-controlled study of durvalumab in combination with gemcitabine plus cisplatin in patients with advanced biliary tract cancer: TOPA-1. . 2022 ASCO GI Cancers Symposium 2022;Abstract 378.
21. Burris HA, Okusaka T, Vogel A, Lee MA, Takahashi H. Patient-reported outcomes for the phase 3 TOPAZ-1 study of durvalumumab plus gemcitatbine and cisplatin in advanced biliary tract cancer. American Society of Clinical Onology (ASCO) Gastriointestinal Cancers Symposium. San Francisco2020.