Dermatology
Maui Derm Hawaii 2023
New Oral Drug Class Highly Specific for Moderate-to-Severe Plaque Psoriasis
Maui – Of the growing list of targeted treatments for moderate-to-severe psoriasis, tyrosine kinase 2 (TYK2) inhibitors are demonstrating uncommon specificity for the underlying pathway of inflammation, according to an update on this novel drug class at Maui Derm 2023. The target, TYK2, mediates several cytokines most closely associated with the inflammation that drives psoriasis. In the phase 3 trials, POETYK PSO-1 & POETYK PSO-2, that led to approval of the first TYK2 inhibitor, rates of efficacy on rigorous endpoints were high and off-target effects were uncommon. New data show no loss of benefit in follow-up out to 2 years. The specificity of action differentiates TYK2 inhibitors from other targeted therapies, including other Janus kinase (JAK) inhibitors.
Unlike injectable biologics that inhibit one or more cytokines that drive inflammation, small molecule oral therapy drugs act at an earlier point in the inflammatory cascade, according to Dr. April W. Armstrong, Professor of Dermatology, University of Southern California, Los Angeles. While signal transductions mediated by the JAK family of intracellular enzymes affect multiple physiologic activities, inhibition of specific JAK enzyme subtypes offer an opportunity for highly targeted effects.
For an exclusive interview with Dr. Melinda Gooderham on the impact to clinical practice, click here
JAK Subtype Importance
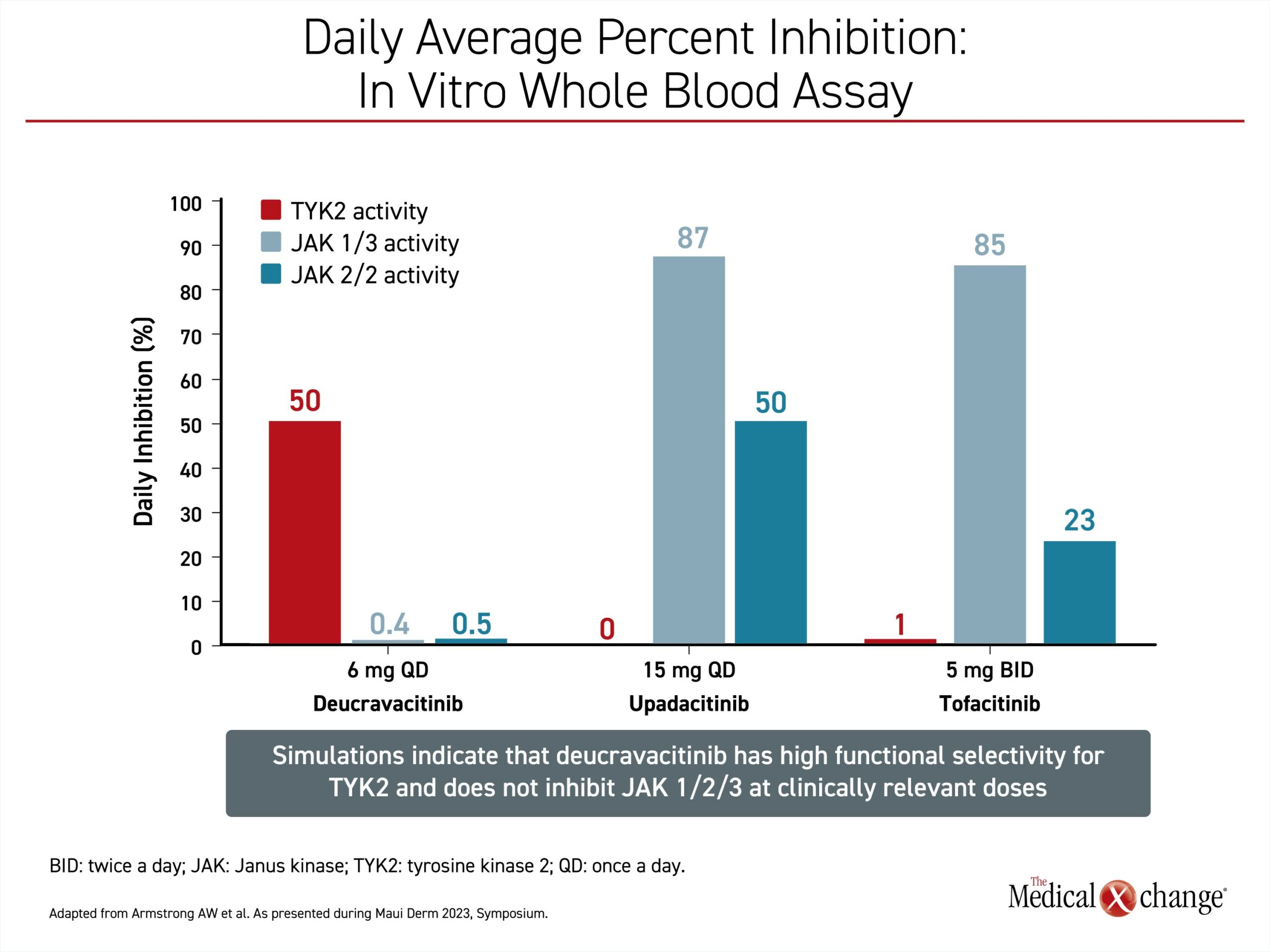
“The value of these drugs is that TYK2 is responsible for mediating signaling of several cytokines that drive psoriasis,” Dr. Armstrong said. The mechanism of TYK2-specific inhibitors is sufficiently different from drugs with specificity on other JAK enzyme subtypes, to such an extent that she considers them unique. The recently approved first-in-class TYK2 inhibitor, deucravacitinib, has little to no effect on JAK1, JAK2, or JAK3. While inversely, conventional JAK inhibitors, such as tofacitinib, have essentially no activity on TYK2 (Figure 1).
“The value of these drugs is that TYK2 is responsible for mediating signaling of several cytokines that drive psoriasis.”
“In particular, it now appears that it is important to avoid JAK2 inhibition, a mediator of many of the off-target effects associated with other JAK inhibitors,” Dr. Armstrong said.
TYK2 Inhibition: Improving Specificity
This same point was reiterated by Dr. Linda F. Stein Gold, Director of Research and the Head of the Division of Dermatology, Henry Ford Health, Detroit, Michigan. Also speaking during the Maui Derm TYK2 symposium, Dr. Stein Gold characterized TYK2 as an “outsider” in relation to the other three major JAK enzyme subtypes. TYK2 specificity means “there are fewer off-target metabolic activities we need to worry about,” she said, citing the clinical studies to support this contention.
“[TYK2 specificity means] there are fewer off-target metabolic activities we need to worry about.”
TYK2 signal transduction is linked to type 1 interferons (alpha and beta) as well as to Th17 differentiation. The latter target is credited with suppression of the interleukin-17 (IL-17), IL-12 and IL-23 cytokines, all of which are known to be important to the pathogenesis of psoriasis.
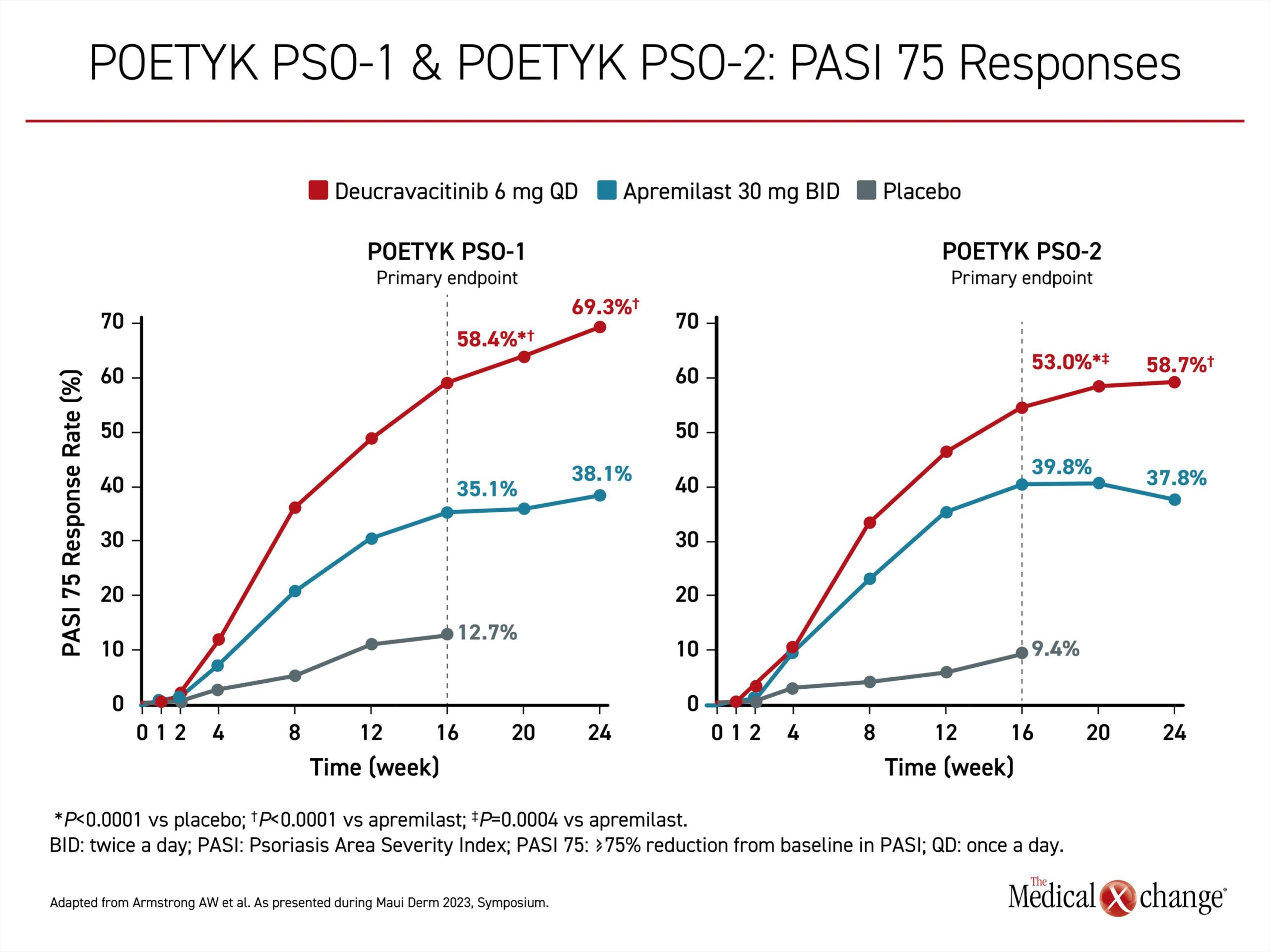
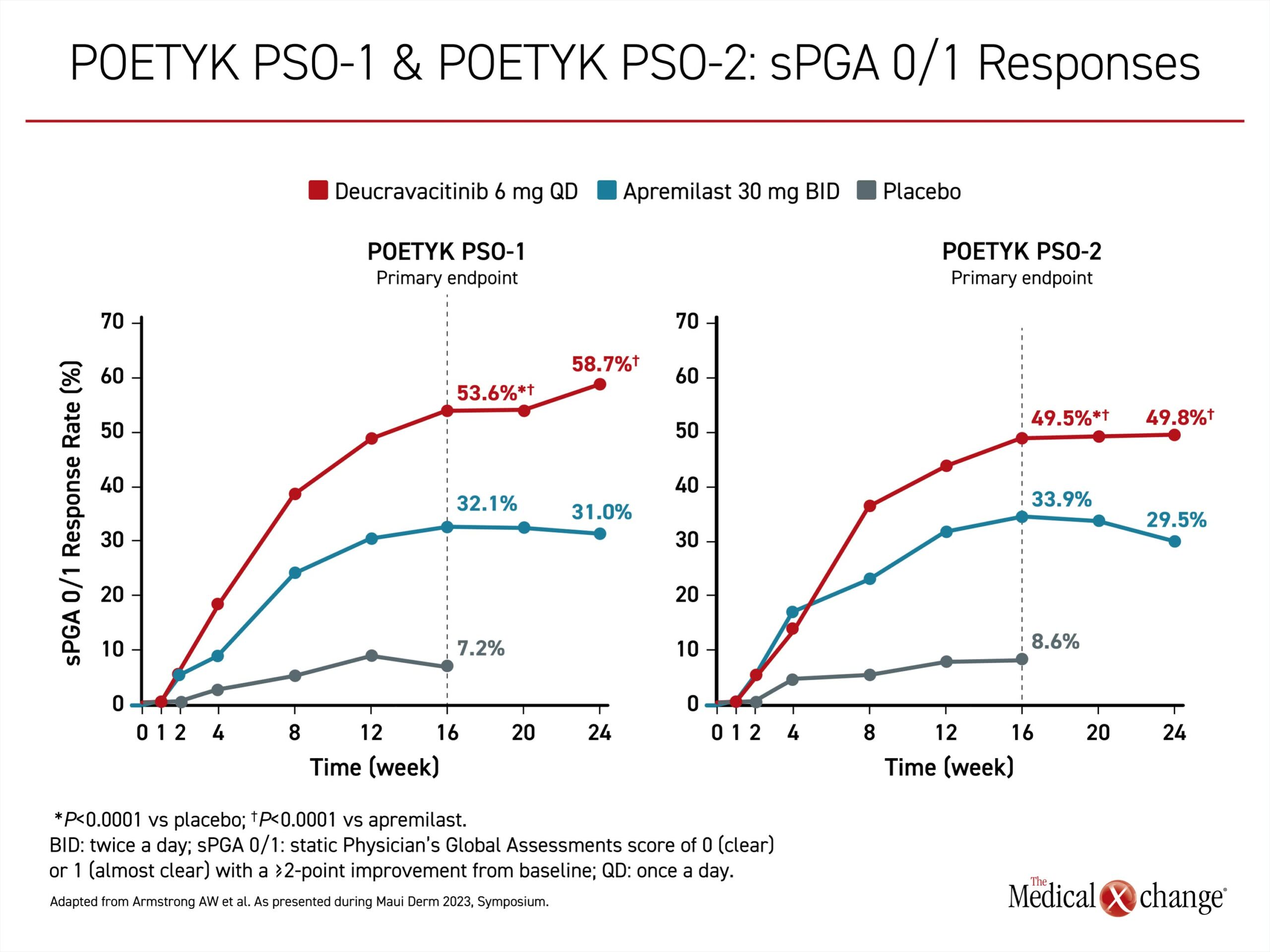
Regulatory approval of the first TYK2 inhibitor, deucravacitinib, was for moderate-to-severe disease in patients inadequately controlled on topical agents and is based on two phase 3 multinational trials; POETYK PSO-1 and POETYK PSO-2. They were similarly designed. In both, patients with moderate to severe psoriasis were randomized in a ratio of 2:1:1 to 6 mg deucravacitinib once daily, 30 mg apremilast twice daily, or placebo. Apremilast, an active comparator, is a phosphodiesterase-4 (PDE4) inhibitor approved for plaque psoriasis. The relatively rigorous co-primary endpoints were Psoriasis Area and Severity Index (PASI) 75 and static Physicians Global Assessment score of 0, meaning completely clear, or 1 (sPGA 0/1), meaning almost clear.
The advantage of the TYK2 inhibitor over both placebo and the active comparator emerged 4 to 6 weeks into the trial. By 16 weeks, deucravacitinib was significantly more effective than both placebo and the active comparator for PASI 75 and sPGA 0/1. At 24 weeks, when response curves in the deucravacitinib arm were still maintaining an upward slope, the proportion reaching PASI 75 was about 70% and 60% higher for deucravacitinib relative to apremilast in POETYK PSO-1 and POETYK PSO-2 respectively (Figure 2). The advantage for the sPGA 0/1 response was of a comparable magnitude (Figure 3).
Phase 3 Trials Align: Significant Results
“The similarity in the response rates for these relatively rigorous endpoints was mutually reinforcing,” according to Dr. Armstrong, who was the principal investigator of POETYK PSO-1. Both trials were published in the January 2023 issue of the Journal of the American Academy of Dermatology (J Am Acad Dermatol 2023;88:29-39/J Am Acad Dermatol 2023;88:40-51).
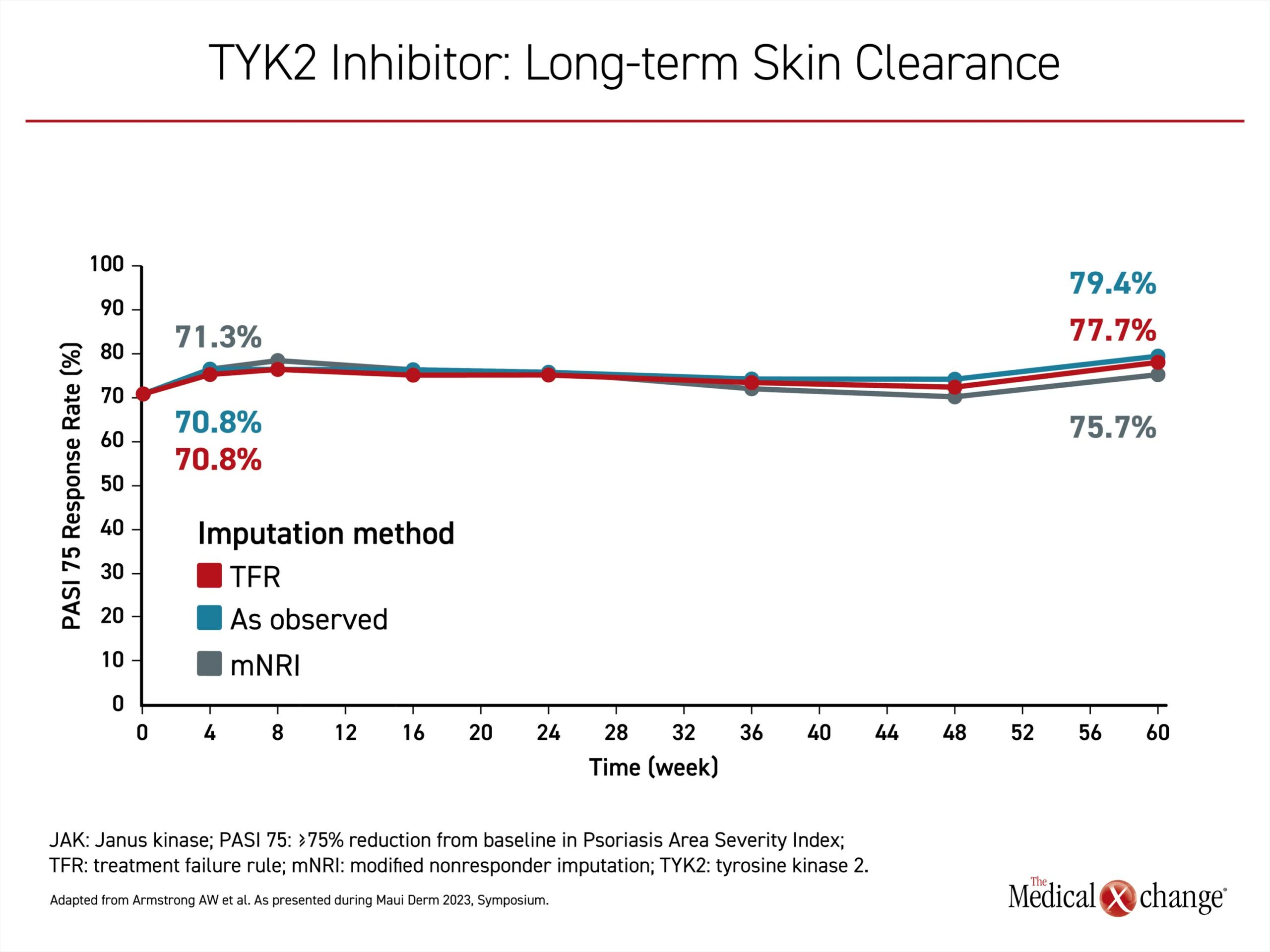
In a phase 3, open-label, long-term extention (LTE) trial, POETYK PSO-LTE, response has been sustained in follow-up to date.Sixty weeks after completion of the trials, the proportion of patients achieving PASI 75 was slightly greater than that observed at the end of the controlled comparison across three methodologies of analysis (Figure 4). Without a direct comparison to other targeted therapies in the long-term extension, there are limited conclusions to be drawn about a greater relative sustained response, but loss of response over time is common on cytokine-targeted monoclonal antibodies.
Allosteric Replacing Catalytic TYK2 Inhibition
In the development of TYK2 inhibitors, deucravacitinib represented a reorientation. Initially, the catalytic domain of TYK2, which has dimer structure, was targeted. Two initial TYK2 inhibitors, brepocitinib and ropsacitinib, which targeted the catalytic domain, reached phase 2 trials. However, these failed to achieve adequate selectivity. The problem was that the catalytic domain is highly conserved across the JAK family, according to Dr. Armstrong.
As a result, attention switched to the regulatory domain of the dimer structure. By targeting a domain not shared by other JAK subtypes, deucravacitinib and other TYK2 inhibitors currently in development imposed very little activity on other JAK enzyme subtypes. According to Dr. Armstrong, the specificity of deucravacitinib to TYK2 is at least 100-fold greater than it is for JAK3 and at least 2000-fold greater than it is for JAK 2 in the experimental setting. The advantage relates to both activity and safety.
So far, this premise is supported by similar analyses performed with other selective allosteric TYK2 inhibitors; NDI-034858 and VTX958. Both are currently in development and have completed phase 2 studies. While each was associated with substantial activity against psoriasis, they were also associated with little or no off-target inhibition of other JAK enzymes. Phase 3 trials with both agents are anticipated based on the evidence so far of clinically viable therapies.
The TYK2 inhibitors in development, along with deucravacitinib, “are seeing a safety profile consistent with a highly specific TYK2 inhibition,” Dr. Armstrong said. In particular, there has been no signal of potential cardiovascular or thrombotic events observed with any of the TYK2 inhibitors approved or now in development. None of these drugs are expected to share the current labeling of less specific JAK inhibitors that now warns of vascular risks.
Rate of Adverse Events Is Low
In the phase 3 trials with deucravacitinib, the rate of adverse events overall was low and similar to both placebo and apremilast. Nasopharyngitis and upper respiratory tract infections were the most common adverse events in all of the study arms. In POETYK PSO-1, for example, these events were reported in 25.4% and 24.3% of deucravacitinib and apremilast patients, respectively (14.7% in the placebo group). Headache (8.6% vs. 21.7%), diarrhea (7.3% vs. 17.6%) and arthralgia (1.7% vs. 19.9%) were lower on deucravacitinib than apremilast. Overall, treatment-related adverse events were lower on deucravacitinib (33.1%) than either apremilast (46.9%) or placebo (45.2%). Herpes zoster infections over 52 weeks of follow-up were higher on deucravacitinib (1.2%) than on apremilast or placebo (0% for both), but low overall.
“There was no signal of an increased risk for MACE [major adverse cardiovascular events] or VTE [venous thromboembolism] in either phase 3 study,” Dr. Armstrong reported.
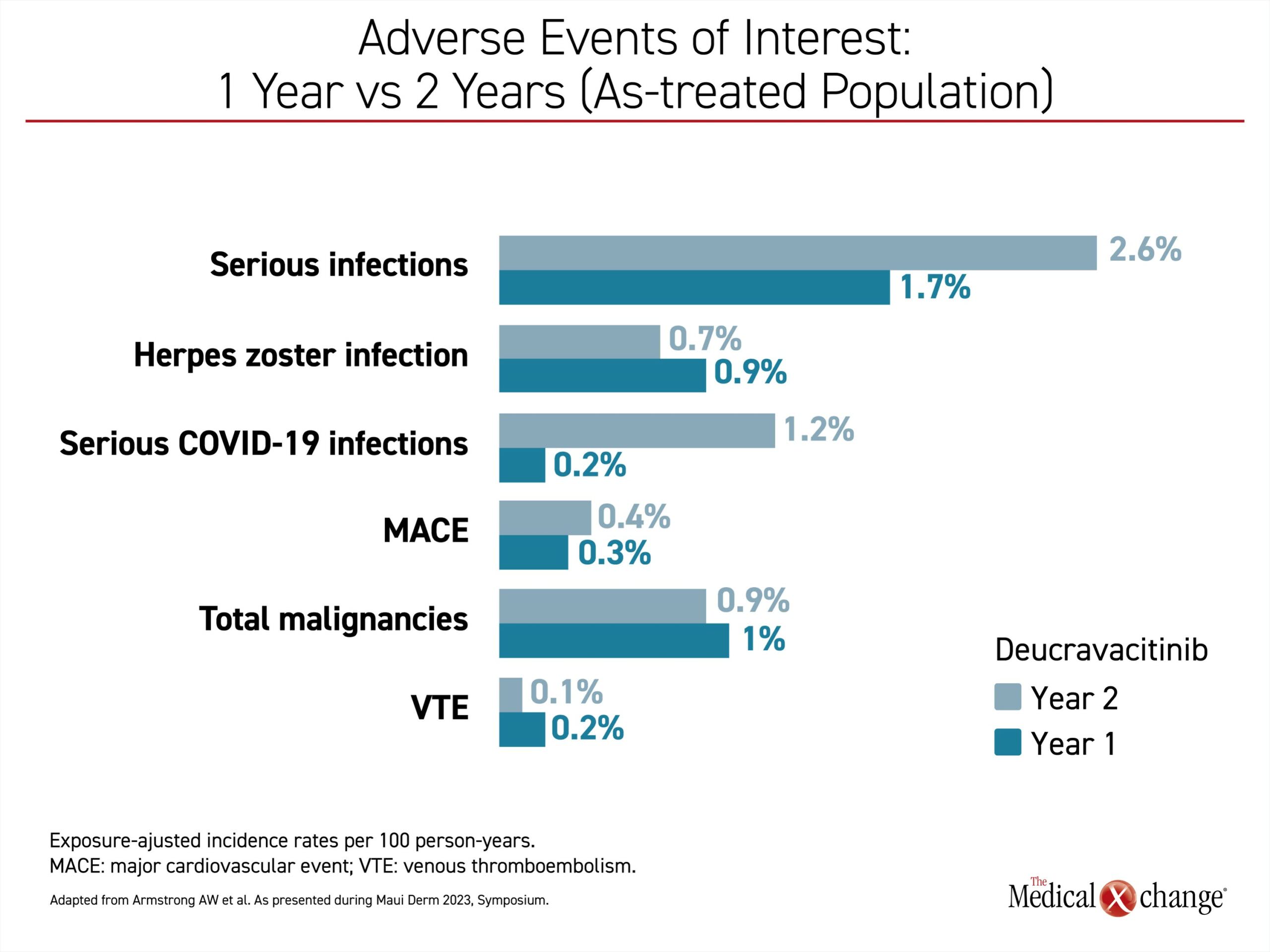
In the long-term follow-up, Dr. Armstrong provided data to show that adverse events in the second year were generally lower relative to the first year. Independent of the higher rate of COVID-19 infections in the second year, the only exception was a higher rate of respiratory infections (Figure 5).
TYK2 Inhibitors in Development Reinforce Specificity
Although data with the selective allosteric TYK2 inhibitors now in development are more limited than those available for deucravacitinib, Dr. Armstrong said the same general type of safety profile has been observed so far. She specifically cited data from a controlled trial with NDI-034858. Infection rates were higher on this agent than placebo (14% vs. 0%), but serious adverse events were uncommon. The low rate of laboratory abnormalities parallels the experience with deucravacitinib in the phase 3 trials. “At 2 years, laboratory abnormalities were seen in a low number of deucravacitinib patients, and when they occurred there was usually a reason,” Dr. Armstrong said, referring, for example, to the low rate of creatine phosphokinase (CPK) elevations that were traced with further inquiry to be observed after strenuous activity.
Novel Oral Therapy Offers Practical Advantages
The approved indication of deucravacitinib is for moderate-to-severe disease in patients inadequately controlled on topical agents. According to Dr. Stein Gold, moderate-to-severe disease typically refers to a body surface area involvement of 10% or greater or to lesions that are disfiguring, such as those on the face. In these patients, deucravacitinib can be considered either as a first systemic therapy or after other targeted agents have failed, she said.
“Patients do not have to be naïve to systemic therapies, including biologics,” according to Dr. Stein Gold. The efficacy of deucravacitinib as a first- or second-line agent is reflected in the phase 3 trials, in which 60% of patients were experienced with monoclonal antibodies or other systemic therapies.
Rather than placing TYK2 inhibitors in front of biologics, Dr. Armstrong suggested these agents are reasonable alternatives to biologics. She said, “We are seeing a level of efficacy that is quite similar to our injectable drugs. When I discuss the options with patients, I do talk about preferences regarding oral versus injectable agents while discussing the safety profiles of the available agents,” she added.
“We are seeing a level of efficacy with TYK2 inhibitors that is quite similar to our injectable drugs.”
For Dr. Stein Gold, there are several practical advantages of deucravacitinib. In addition to an oral therapy, which she said many patients prefer, deucravacitinib is not associated with any drug/drug interactions, which can simplify prescribing for individuals with multiple comorbidities. She pointed out that once daily deucravacitinib can be taken with or without food.
In patients who opt for deucravacitinib, Dr. Armstrong recommended screening for tuberculosis, verifying that patients have received age-appropriate immunizations, including protection from herpes zoster, and obtaining baseline liver function tests for those with known or suspected liver disease. Although she recommended ongoing surveillance for infectious diseases in patients on maintenance deucravacitinib treatment, she said that no routine laboratory monitoring is needed without cause.
Overall, the specificity of TYK2 inhibitors for control of psoriasis-associated inflammation validated in the clinical studies with deucravacitinib has been supported by the ongoing studies with TYK2 inhibitors in development.
Conclusion
In an update on the relative role of TYK2 inhibition in the control of psoriasis, the specificity of the first-in-class deucravacitinib was emphasized in relationship to its clinical benefits and its safety. With anti-psoriasis activity that rivals injectable agents, the particular advantage of this agent is derived from its selectivity, a characteristic relevant to its anti-inflammatory effect and favourable safety profile. Now approved in Canada, deucravacitinib, will offer a novel once-daily oral option for patients inadequately controlled on topical agents.