Hematology
Allogeneic Stem Cell Transplantation: Expert Review and Commentary
Conditioning for Allogeneic Hematopoietic Stem Cell: Strategies Compared
Mattias Stelljes, MD
Professor of Medicine
Head of the Adult Allogeneic Stem Cell program
University of Münster
Münster, Germany
Thomas J. Nevill, MD
Clinical Professor of Medicine
Division of Leukemia and Bone Marrow Transplant
Vancouver General Hospital
Vancouver, British Columbia
Victor Lewis, MD
Director of the Blood and Marrow Transplant Program
Alberta Children’s Hospital,
Calgary, Alberta
Prior to final results from a phase 3 trial, efficacy and safety differences between the conditioning agents treosulfan and busulfan were assumed by many to be modest. The first comparative trial (MC-FludT.14/L), which has now been published, associated treosulfan with lower rates of toxicity and a higher rate of overall survival (OS) at 36 months of follow-up. The trial was conducted in adults undergoing allogeneic stem cell transplantation (AlloSCT) for acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS). Despite a design to show treosulfan was non-inferior to busulfan when each was combined with fludarabine, a superiority analysis confirmed the advantages of treosulfan at 36 months of follow-up. In this current review, Dr. Stelljes, one of the trial coauthors, describes the background of the comparative trial and its clinical impact in Germany. Dr. Nevill and Dr. Lewis provide a Canadian perspective on these conditioning agents, discussing them in the context of adult and pediatric patients, respectively.
Dr. Stelljes: Background for the Phase 3 Trial Comparing Conditioning Regimens
For many hematologic malignancies, AlloSCT is essential in treatment with curative intent. Conditioning regimens play a fundamental role in the success of AlloSCT by reducing tumour burden and improving stem cell engraftment. Whether the conditioning intent is myeloablative at full (MAC) or reduced-intensity (RIC) levels, the therapeutic window is generally narrow and reached by balancing the competing goals of preventing relapse while avoiding life-threatening toxicity. The optimal intensity of conditioning regimens, which is defined by its components and adjusted to remain within the therapeutic window by disease- and patient-related factors, continues to be a major focus in a quest to improve outcomes.
In stem cell transplant conditioning regimens, the bifunctional alkylating agent busulfan has been used in combination with other agents, such as fludarabine and cyclophosphamide, for several decades.1 Treosulfan, the newer of these two conditioning agents, is a hydrophilic analogue of busulfan.2 Unlike busulfan, treosulfan is a prodrug activated in vivo through a non-enzymatic process that does not require hepatic metabolism. Treosulfan was granted orphan drug status in Germany in 2004. An indication for use in AlloSCT conditioning was issued by the European Medicines Agency subsequently. In Canada, regulatory approval for a conditioning indication was granted in 2021.
On the basis of non-comparative studies, busulfan and treosulfan were both considered to be effective options within conditioning regimens involving additional cytotoxic drugs or additional modalities, such as radiation. From its development, treosulfan has been proposed as a potentially better-tolerated alternative to busulfan, but most early studies were uncontrolled and the exact regimens and patient characteristics varied among the studies. As a result, differences between busulfan and treosulfan for clinical outcomes were speculative until the comparative phase 3 trial was conducted.
In this multicenter trial (MC-FludT.14/L), 570 patients with AML or MDS scheduled for AlloSCT were randomized to 30 g/m2 treosulfan or 6.4 mg/kg of busulfan. Both agents were combined with standard dosages of fludarabine. Patients ranged in age from 50 to 70 years or patients <50 years of age with a comorbidity index (HCT-CI) of >2 and were considered ineligible for a MAC regimen. The stated objective of the study was to demonstrate non-inferiority of treosulfan to busulfan. The primary efficacy endpoint was event-free survival (EFS) with events defined as absence of disease recurrence, graft failure, or death from any cause.
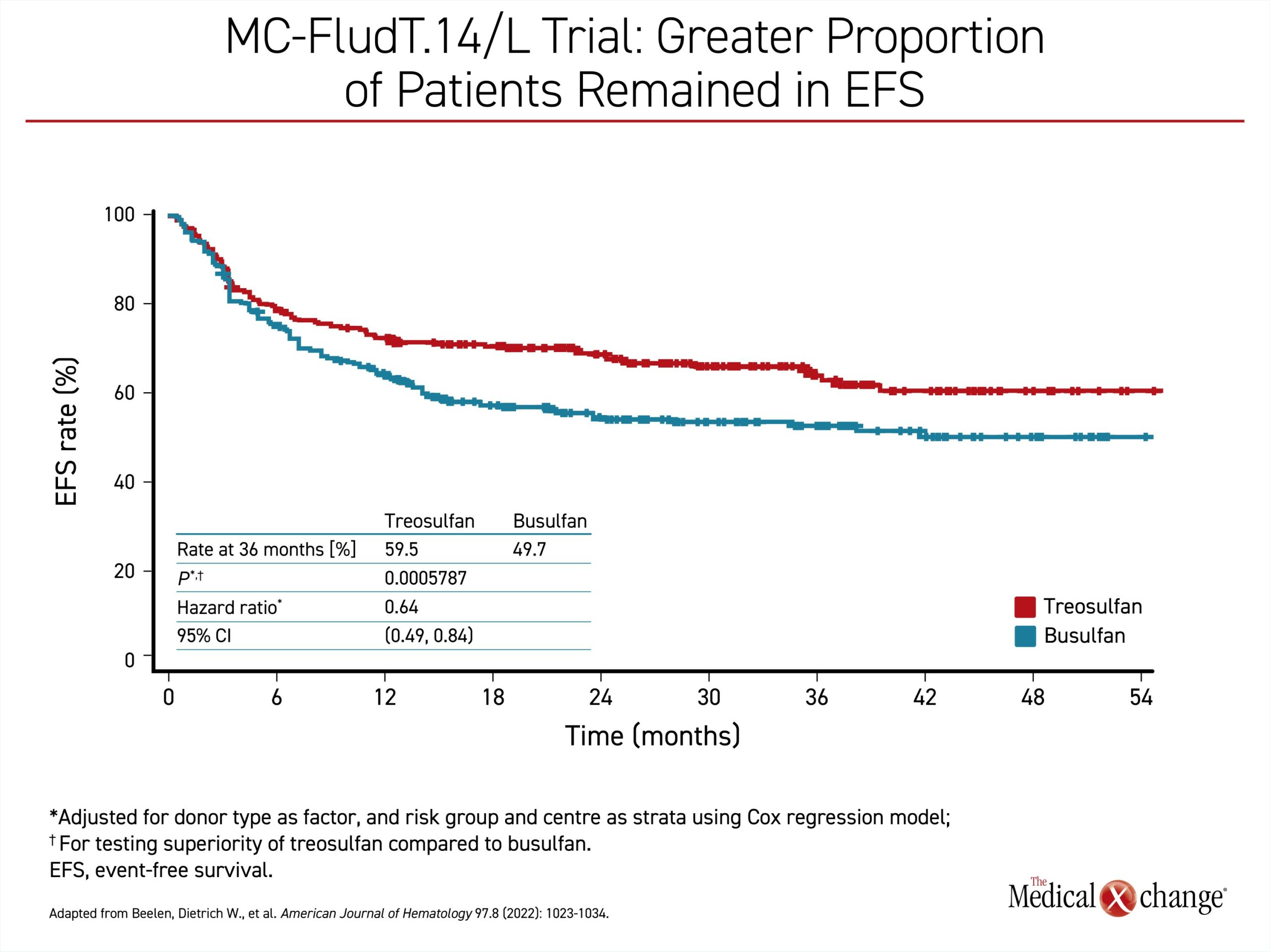
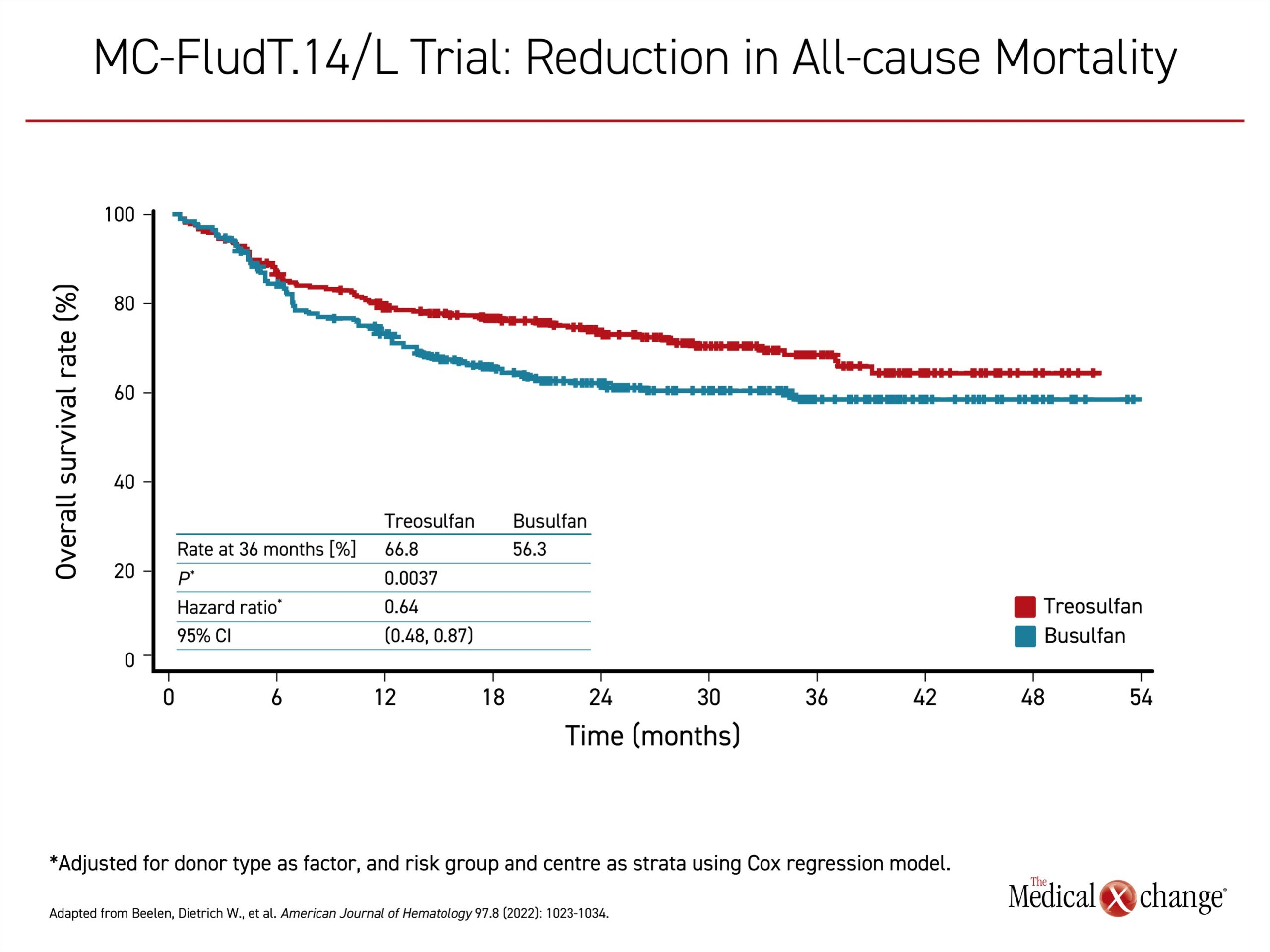
In the interim analysis released several years ago and published in 2020,3 treosulfan met the study definition of non-inferiority, but the advantage of treosulfan for several key outcomes, including EFS, allowed an analysis for superiority, which was explored in the subsequently published final results. With 36 months of follow-up,4 the greater proportion of treosulfan patients that remained in EFS (59.5% vs. 49.7%) translated into a 36% lower risk of graft failure, recurrence, or death (OR 0.64; P=0.0006) (Figure 1). The rate of OS at 3 years (66.8% vs. 56.3%) associated the treosulfan-based regimen with a similar relative risk advantage for survival (OR 0.64; P=0.0037) (Figure 2). Graft failures were uncommon in both groups (0.4% vs. 3.2%) but higher in the group receiving busulfan. The rates of chronic graft-versus-host disease (GvHD) and treatment-emergent adverse events, including serious events, were similar in the two arms.
Although the significant advantages for the treosulfan-based regimen on the primary outcome had not been anticipated, the interim results had already changed practice at participating trial sites even before the most recent publication of the long-term outcomes. In AML and MDS candidates for AlloSCT at these centers, including our own, busulfan has been largely replaced by treosulfan. Even though treosulfan-based regimens cannot be characterized as a standard in Europe at this time, busulfan has a more diminished role in our center and many others. In AlloSCT with curative intent in AML and MDS, the phase 3 study demonstrated that treosulfan, which is generally better tolerated than busulfan, is associated with better long-term outcomes.
At the 2022 annual meeting of the American Society of Hematology (ASH), we presented subsequent data on treosulfan-based regimens in MDS, including the experience in patients with high-risk disease.5 The outcomes in this real-world analysis were generally consistent with those of the comparative trial, with low rates of relapse (15%) and non-relapse mortality (10%) at 2 years. Although OS at 2 years was lower in the subgroup of patients with very high-risk disease relative to those with high-risk disease (55% vs. 81%), the age, donor type, comorbidity index, pre-conditioning, or bone marrow blast count did not have an impact on survival. This suggested to us that survival is linked to cytogenic and molecular features of disease rather than to disease burden defined by bone marrow blast count.
Dr. Nevill: Evolving AlloSCT Conditioning Regimens for Adult Patients in Canada
Unlike the growing consensus on preferred treatment options for hematological diseases with specific prognostic features, there is substantial variability in the AlloSCT regimens even within MAC or RIC protocols. We became interested in treosulfan because of the potential for improved tolerability in patients with comorbidities, particularly those with significant hepatic dysfunction, but this agent is not yet a standard in Canada.
Among adult patients who are candidates for busulfan-based conditioning, treosulfan is a relatively new alternative in Canada. Compared to the European countries, such as Germany and Italy, where most of the trials have taken place, treosulfan was an experimental agent until it was licensed by Health Canada two years ago.
The phase 3 study provides a basis for comparison, but there are many variables to consider when individualizing conditioning regimens. Dose equivalence between busulfan and treosulfan is not clearly defined, so the doses compared in the phase 3 trial (6.4 g/m2 vs. 30 mg/kg for busulfan and treosulfan, respectively) do not necessarily represent optimum dosing for patients with different levels of risk for toxicity and relapse. The doses of treosulfan in a MAC or RIC conditioning regimen are not that different, but there is growing interest in titrating conditioning regimens for adequate myeloablative effect with a lower relative risk of adverse events. This is a major reason why we have moved away from radiation for AlloSCT conditioning for non-lymphoid malignancies.
It was not clear from the phase 3 trial whether the improved outcomes associated with treosulfan related to improved tolerability or greater myeloablative effect, but we and others are employing this agent in a growing proportion of cases. Treosulfan is not yet a standard. The decision to use a treosulfan-based conditioning regimen is individualized, but it is more commonly considered at our center.
Dr. Lewis: Evolving AlloSCT Conditioning Regimens for Pediatric Patients in Canada
Relative to AlloSCT in adults, pediatric AlloSCT conditioning protocols have emerged through largely independent studies and empirical experience. Our interest in treosulfan developed in 2010, when we were the first to import this agent into Canada. Our interest was based on European data suggesting that this drug had a lower toxicity compared to busulfan. Our results with busulfan in immune deficiency patients at the time were unacceptable with substantial treatment-related mortality, and we were impressed with treosulfan as an alternative. It was better tolerated without any apparent increase in risk of engraftment failure.
In AlloSCT conditioning for non-malignant indications, treosulfan has become our standard. It is also our first choice for AlloSCT in non-malignant diseases for those patients undergoing haploidentical transplants. However, busulfan otherwise remains our standard for malignant indications. In malignant disease where the goal of conditioning for AlloSCT includes elimination of minimal residual disease as well as improving the opportunity for engraftment, we do not yet have sufficient follow-up data to confirm that there is no increase of relapse with a potentially less aggressive regimen. It is important to note that this is an institutional choice and does not preclude moving to treosulfan-based regimens for AlloSCT conditioning in the treatment of non-haploid-identical malignant disease, but data are needed.
In the non-malignant setting, the major advantage of treosulfan is the lower relative risk of significant toxicities and their complications. Mucositis is an example. With busulfan, nasogastric tubes or total parental nutrition are frequently needed for the often severe inflammation in the mouth and esophagus. With treosulfan, mucositis still occurs but it is typically less severe and of shorter duration, so the problems with sustaining adequate nutrition are often avoided. When we use treosulfan-based conditioning, we find that hospital stay is usually shorter.
The advantages of treosulfan-based conditioning are now relatively well recognized. Although uptake of this approach was slow at first elsewhere in Canada, it is now the agent of choice at many transplant centers for malignant and non-malignant pediatric indications. While we once had to obtain authorization for treosulfan, this agent is now on our formulary and is now easily accessed for routine indications.
Summary
Treosulfan-based conditioning regimens for AlloSCT have supplanted those based on busulfan at many centers in Europe, encouraged by a phase 3 trial that associated the newer alkylating agent with significantly superior OS rates after three years of follow-up. In Canada, where treosulfan was relatively recently approved, experience is more limited in adults but growing. Again, the role appears to be as a better-tolerated alternative to busulfan with superior clinical outcomes for patients undergoing AlloSCT for AML or MDS. In children, the tolerability advantage of treosulfan-based conditioning was the basis of initial interest. At several centers, including the University of Calgary where it was first evaluated in Canada, it is now a standard for non-malignant AlloSCT.
References
- Galaup A, Paci A. Pharmacology of dimethanesulfonate alkylating agents: busulfan and treosulfan. Expert Opin Drug Metab Toxicol 2013;9(3):333-47. DOI: 10.1517/17425255.2013.737319.
- Romanski M, Wachowiak J, Glowka FK. Treosulfan Pharmacokinetics and its Variability in Pediatric and Adult Patients Undergoing Conditioning Prior to Hematopoietic Stem Cell Transplantation: Current State of the Art, In-Depth Analysis, and Perspectives. Clin Pharmacokinet 2018;57(10):1255-1265. DOI: 10.1007/s40262-018-0647-4.
- Beelen DW, Trenschel R, Stelljes M, et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): a randomised, non-inferiority, phase 3 trial. Lancet Haematol 2020;7(1):e28-e39. DOI: 10.1016/S2352-3026(19)30157-7.
- Beelen DW, Stelljes M, Remenyi P, et al. Treosulfan compared with reduced-intensity busulfan improves allogeneic hematopoietic cell transplantation outcomes of older acute myeloid leukemia and myelodysplastic syndrome patients: Final analysis of a prospective randomized trial. Am J Hematol 2022;97(8):1023-1034. DOI: 10.1002/ajh.26620.
- Floeth M, Beckmann E, Reicherts C, Marx J, Stelljes M. Treosulfan-based conditioning prior to allogeneic hematopoietic cell transplantation (alloHCT) for patients with myelodysplastic syndrome (MDS): promising survival outcome including patients with high-risk disease. Blood 2022;140:Supplement 1:7587-7588.