Cardiology
10th European Stroke Organisation Conference (ESOC) 2024
Balancing Relative Risk of Poor Functional Outcomes Guides Factor Xa Inhibitor Reversal Agent Selection
Basel – The pivotal ANNEXA-I trial, which was first reported at the World Stroke Congress (WSC) last October, was stopped early, demonstrating that a specific reversal agent was more effective than usual care for controlling factor Xa (FXa) inhibitor-induced intracerebral hemorrhages (ICH). In new secondary analyses presented at ESOC 2024, the favourable balance of benefits to risks was outlined in more detail. For the primary result in the ANNEXA-I trial, the reversal agent improved outcomes by preventing hematoma expansion. A new and more detailed analysis demonstrates that the risks imposed by hematoma expansion far exceed those of thrombotic events. Both adverse events increase mortality, but the data show hematoma expansion is the more important target, both for mortality reductions and for reducing the risk of disability.
ANNEXA-I: Recently Published Data
In the multinational ANNEXA-I trial, which was recently published in the New England Journal of Medicine, andexanet alfa was found to be more effective than usual care in preventing adverse outcomes among patients with FXa inhibitor-associated ICH. The primary endpoint was hemostatic efficacy, defined by expansion of the hematoma volume by 35% or less at 12 hours after baseline; a change in National Institutes of Health Stroke Scale (NIHSS) score of <7 points at 12 hours; and no rescue therapy 3 to 12 hours after randomization. Overwhelming evidence that the reversal agent prevents expansion of the hematoma resulted in early termination of the trial.
For an exclusive interview with Dr. Andrew Demchuk on the impact to clinical practice, click here
The Balance of Benefit to Risk
Since their introduction, FXa inhibitors, such as apixaban and rivaroxaban, have proven highly effective for reducing the risk of thrombotic events but at a modest cost of an increased risk for hemorrhage, including ICH. Andexanet alfa provides an opportunity to control these events. Although the competing risk of thrombosis is well recognized, there were only 13 of these events in the ANNEXA-I study or about one-quarter of the incidence of clinically meaningful hematoma expansions.
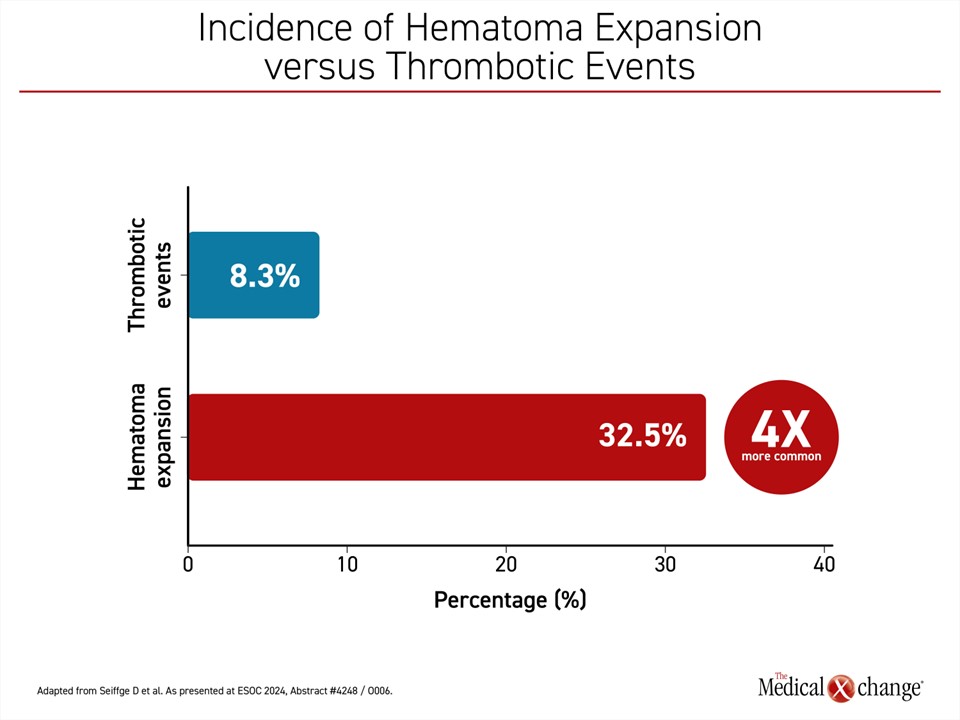
Thrombotic events and death were monitored as major safety endpoints, and the latest secondary analyses, presented at this year’s ESOC, confirmed that both hematoma expansion and thrombotic events were independently associated with increased mortality at 30 days. However, hematoma expansion was about four times more common than thrombotic events (32.5% vs. 8.3%) (Figure 1). Moreover, hematoma expansion alone, unlike thrombotic events, produced significant disability as measured with the modified Rankin Score (mRS).
When the interim data were presented at the World Stroke Congress, 452 patients of 530 enrolled were available for efficacy and safety analyses. These patients had presented in an emergency room with an ICH and had taken a FXa inhibitor within the past 15 hours. They were randomized in a 1:1 ratio to andexanet alfa or usual care, which included prothrombic complex concentrate (PCC) in 86% of the control arm.
As previously reported, there was a 13.4% advantage in hemostatic efficacy for the reversal agent relative to usual care. Although thrombotic events were less frequent, they were 4.6% more common in the experimental arm.
One of the secondary analyses examined the clinical consequences of hematoma expansion and thrombotic events. The results reinforce the net benefit of rapidly controlling hematoma expansion with a reversal agent in FXa inhibitor-associated ICH, according to Dr. David J. Seiffge, Senior Attending Physician in the Department of Neurology, Inselspital, University of Bern, Switzerland.
“The results reinforce the net benefit of rapidly controlling hematoma expansion.”
The objective of this analysis of ANNEXA-I was “to inform benefit-to-risk analyses,” by evaluating the clinical consequences of hematoma expansion and thrombotic events on the key outcomes of mortality and disability, stated Dr. Seiffge.
Impact of Hematoma Expansion and Thrombotic Events
The secondary analysis presented by Dr. Seiffge evaluated the two key 30-day outcomes of mortality and disability in relation to hematoma expansion and thrombotic events. Mortality was evaluated through a time-dependent Cox regression model in relation to covariates, such as age, sex, prior myocardial infarction (MI), prior stroke, and congestive heart failure (CHF). Disability was evaluated in a landmark analysis of functional disability, defined as a mRS score ≥4.
For those who developed a hematoma expansion within the first 5 days after randomization, the risk of 30-day mortality was increased by about two-fold (aOR 2.34; 95% CI 1.49 – 3.68) relative to those who did not. For those with a thrombotic event, the risk of 30-day mortality was also increased by about two-fold (aOR 2.11; 95% CI 0.74 – 6.0) relative to those without. Due to the infrequency of thrombotic events in ANNEXA-I, the confidence intervals for the risk were large. There was a significant interaction with older age (P<0.004) and prior CHF (P=0.03) but not with prior MI or stroke. There was a trend for greater risk in females (P=0.087).
For disability, defined as mRS ≥4, thrombotic events were associated with a numerical increase but not a statistical increase in the odds ratio (OR 1.22, 95% CI 0.33 – 4.45; P=0.767). Hematoma expansion, in contrast, nearly doubled the risk of disability (OR 1.93, 95% CI 1.07 – 3.47; P=0.029). None of the covariates were significantly associated with risk of disability with the exception of a high rate of hematoma expansion at the time of a pre-treatment scan.
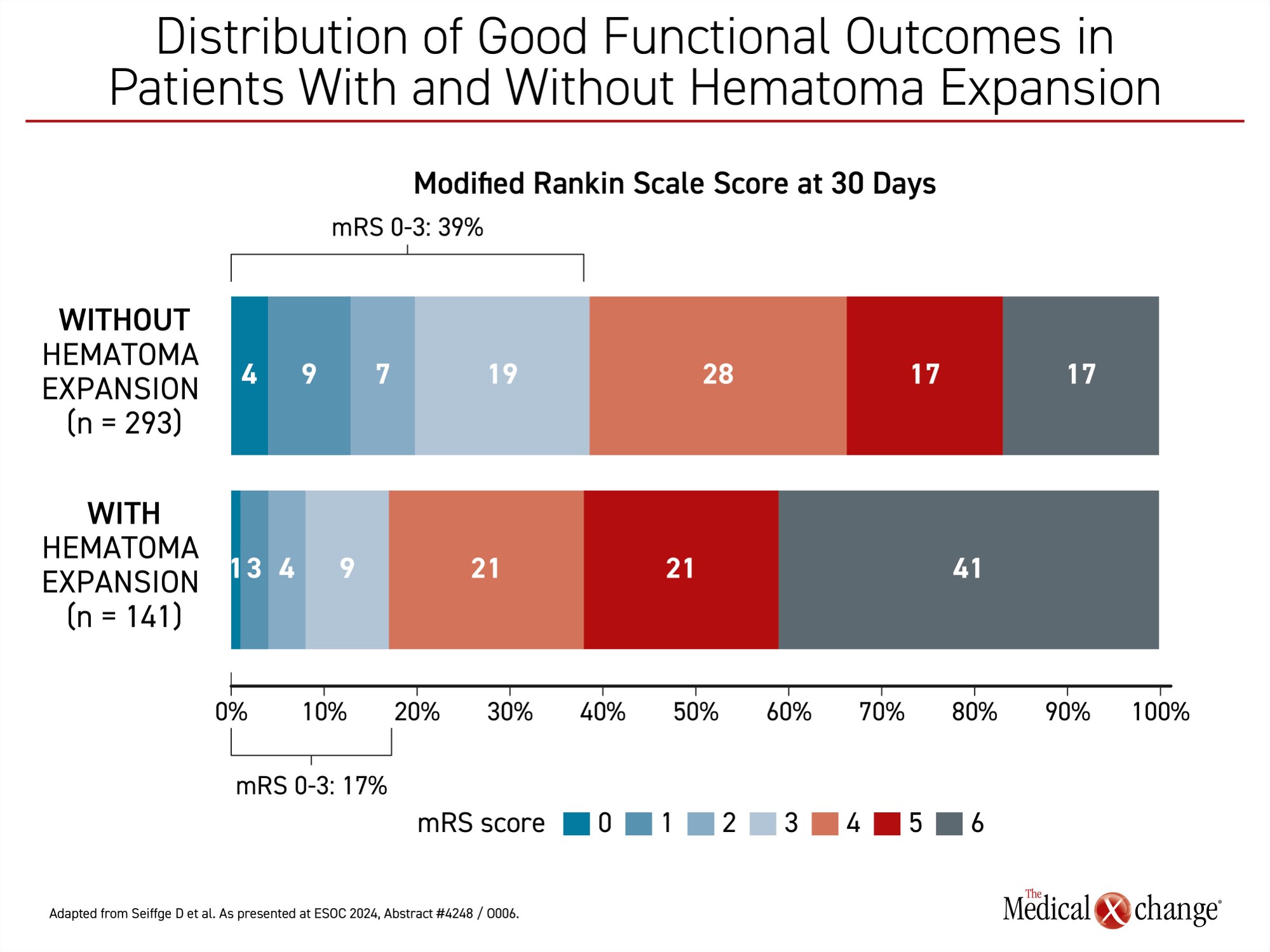
When those with or without thrombotic events were compared, the proportion with good functional outcome at 30 days was nearly identical (32% vs. 31%, respectively). Conversely, when those with or without a hematoma expansion were compared, 39% of those without hematoma expansion had a good functional outcome (mRS <4) versus 17% with hematoma expansion, Dr. Seiffge reported (Figure 2).
Risk of Death Similar for Bleeds and Thromboses
Overall, the data show that the hazard ratio for death at 30 days is similar for those who have a hematoma expansion or those who have a thrombotic event (HR 3.33 vs. 2.98, respectively), but thrombotic events were far less common overall.
The low rate of thrombotic events in the ANNEXA-I trial is a potential limitation of this secondary analysis, according to Dr. Seiffge, but he said these types of detailed analyses are important to provide reassurance that reversal agents for FXa inhibitors have an acceptable benefit-to-risk ratio. Due to the rapid growth in the use of apixaban, rivaroxaban, and edoxaban, and other direct oral anticoagulants (DOACs) the need for reversal agents has grown more urgent.
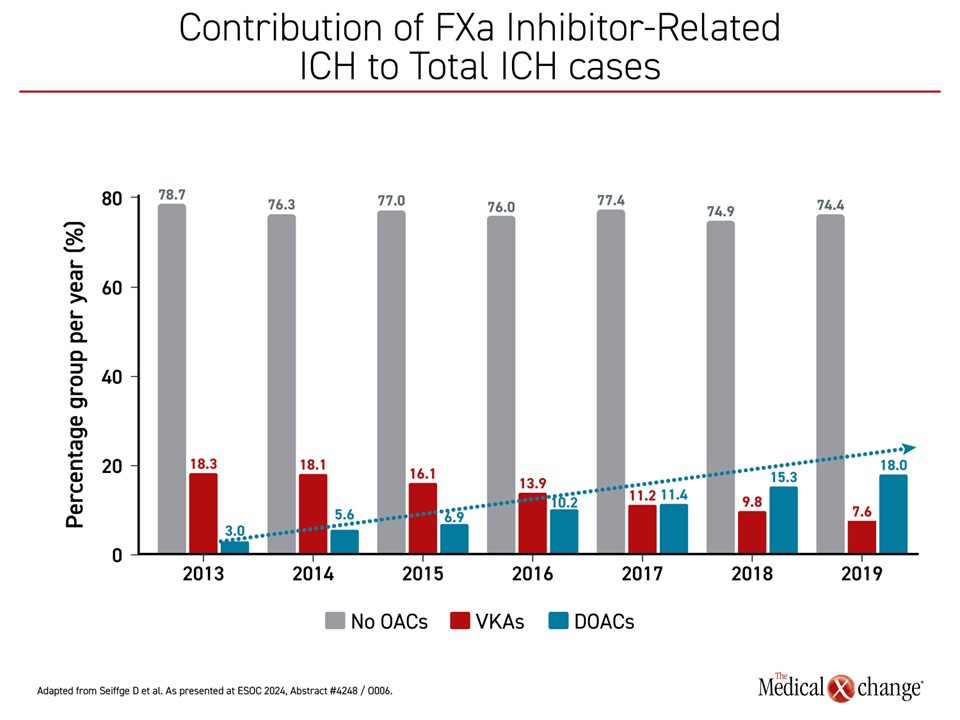
“About 20% of ICH is due to FXa inhibitors,” said Dr. Seiffge, citing European data. On a graph tracing the incidence of ICH since 2013, which has remained relatively stable, the cases attributed to warfarin shrank from about 20% to less than 10%. In contrast, the proportion attributed to DOACs increased from about 3% to near 20%, the current rate (Figure 3).
“About 20% of ICH is due to FXa inhibitors.”
Another analysis also examined the relative benefit-to-risk of the FXa specific reversal agent andexanet alfa by combining ANNEXA-I trial data with data from ANNEXA-4, a previous trial evaluating this reversal agent. In this non-randomized study, also published in the New England Journal of Medicine, andexanet alfa was associated with good or excellent hemostasis within 12 hours in 82% of patients. A thrombotic event occurred in 10% within 30 days of treatment.
When the data were combined, thrombotic events occurred in 10.4% of patients treated with andexanet alfa, presented Dr. Mike Sharma, Chair of Stroke Prevention, McMaster University, Hamilton, Ontario. Of these events, 39 were ischemic strokes, 26 were venous thromboembolisms (VTE), and 22 were MIs.
Using the combined ANNEXA trial data, a logistic regression model was employed to determine the association between baseline characteristics and risk of thrombotic events. Variables included age, history of prior thrombotic events, vascular risk factors, blood pressure, and site of bleeding, which in the case of ANNEXA-4 included the gastrointestinal tract and other organs.
No Predictors of Thrombotic Events Identified
“We did not identify any baseline variables that denoted a higher risk of thrombotic events,” Dr. Sharma reported. While differences would have been expected to “help with the benefit-to-risk calculation” when considering a reversal agent in the event of FXa-inhibitor induced bleeding, he suggested this work is incomplete.
“We did not identify any baseline variables that denoted a higher risk of thrombotic events.”
In a related analysis of arterial thrombotic events and risk of reversal agents, he reported these events did track with higher mortality, but he said that this work is part of several ongoing efforts to refine benefit-to-risk predictors for the sake of quantifying and qualifying the advantages and risks of a FXa inhibitor reversal agent in an individual patient.
Despite efforts to further explore relative risks in patients with ICH or other forms of hemorrhage on a FXa inhibitor, the published studies have unequivocally shown a rapid reversal of FXa inhibitor activity and an associated reduction in hemorrhage risk. In ANNEXA-4, a 92% reduction in anti-FXa activity was observed within hours of administration. By 12 hours, 82% had good or excellent hemostasis.
In ANNEXA-I, when most patients in the usual care arm received PCC, excellent control defined as ≤20% hematoma expansion was substantially increased by andexanet alfa (55.9% vs. 45.3%) as was overall excellent or good control (63.9% vs. 52.4%). Good control was modestly improved (8.0% vs. 7.1%). For the hematoma expansion threshold of ≥12.5%, the relative advantage of andexanet alfa approached 45% (11.6% vs. 19.0%).
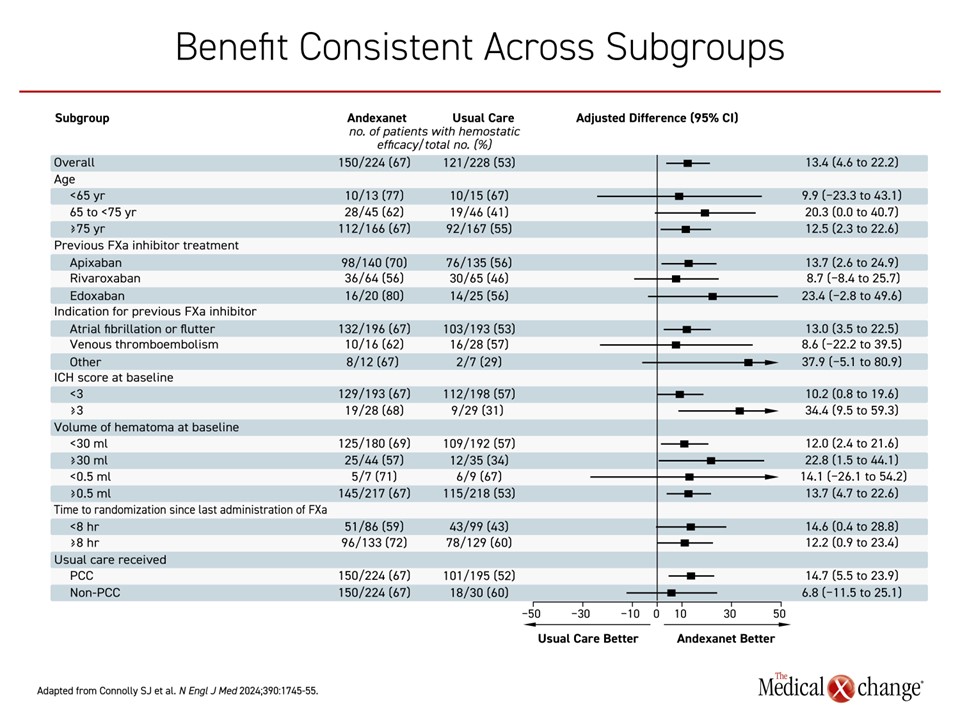
In the analysis by Dr. Seiffge, the impact of major events on outcomes was evaluated independent of treatment, so the impact of significant hematoma expansion on an increased risk of mRS-assessed disability (≥4) was not specific to therapy, but the data underline the clinical value of limiting hematoma expansion both for this endpoint and for mortality. While it is attractive to find independent predictors of hematoma expansion and thrombotic events to improve benefit-to-risk ratios for individual patients, it is important to recognize that there was a consistent and general direction of benefit across subgroups in ANNEXA-I (Figure 4).
Future directions of research might include an evaluation of outcomes relative to serum levels of FXa inhibitor at the time of treatment or the dose of andexanet alfa. For the goal of inhibiting hematoma expansion to prevent ICH-related mortality and morbidity, the most recent analyses support the principle that prompt treatment with andexanet alfa provides net protection against the most devastating consequences of uncontrolled bleeding.
The most recent analyses support the principle that prompt treatment with andexanet alfa provides net protection.
Conclusion
Fulfilling the previously unmet need for a specific FXa inhibitor reversal agent to neutralize the effect of an anticoagulant in case of an ICH or major bleed, andexanet alfa offers significant reductions in the risk of ICH-based complications. This is accompanied by a smaller relative increased risk of thrombotic events. The risk of these events is unequal. In ANNEXA-I, there was a 4-fold greater rate of hematoma expansion events than thrombotic events. Although both are associated with increased mortality, hematoma expansion is more closely related to significant functional disability as defined by mRS ≥4. Ongoing research aims to refine patient selection criteria for optimal outcomes, but the direction of benefit was favourable in almost all subgroups in the all-comer ANNEXA-I enrollment.