Rheumatology
2019 EULAR Congress
Phase 3 Data with New JAK Inhibitor in Rheumatoid Arthritis Predicts Evolving Sequence of Therapies
Madrid – Phase 3 data with a new JAK inhibitor in late stages of clinical development predict a change in the sequence of therapies for rheumatoid arthritis (RA). At the 2019 EULAR Congress, one of a series of studies from a pivotal trials program associated a novel oral JAK inhibitor with greater efficacy than a tumor necrosis factor (TNF) inhibitor delivered by injection. In this head-to-head study, the advantage was sustained to 48 weeks. In a switch substudy of the same trial, both groups of patients with an inadequate response achieved major benefits by switching to the opposite therapy, but benefit was greater when patients were switched from the TNF inhibitor to the JAK inhibitor than the opposite.
The pro-inflammatory JAK/STAT pathway has proven to be a viable target for therapies directed at RA since approval of tofacitinib, the first agent in this class to receive an indication for RA. Newer agents with greater selectivity on JAK enzymes of interest are expanding this class. These include baricitinib, which has been approved by the US FDA, and upadacitinib, which is being evaluated in an extensive phase 3 trials program. Of new trial data presented on upadacitinib at the 2019 EULAR Congress, one involved a head-to-head comparison with the TNF inhibitor adalimumab. The other involved an indirect match-adjusted comparison with the first approved JAK inhibitor, tofacitinib.
SELECT-COMPARE: Latest 48-week Results
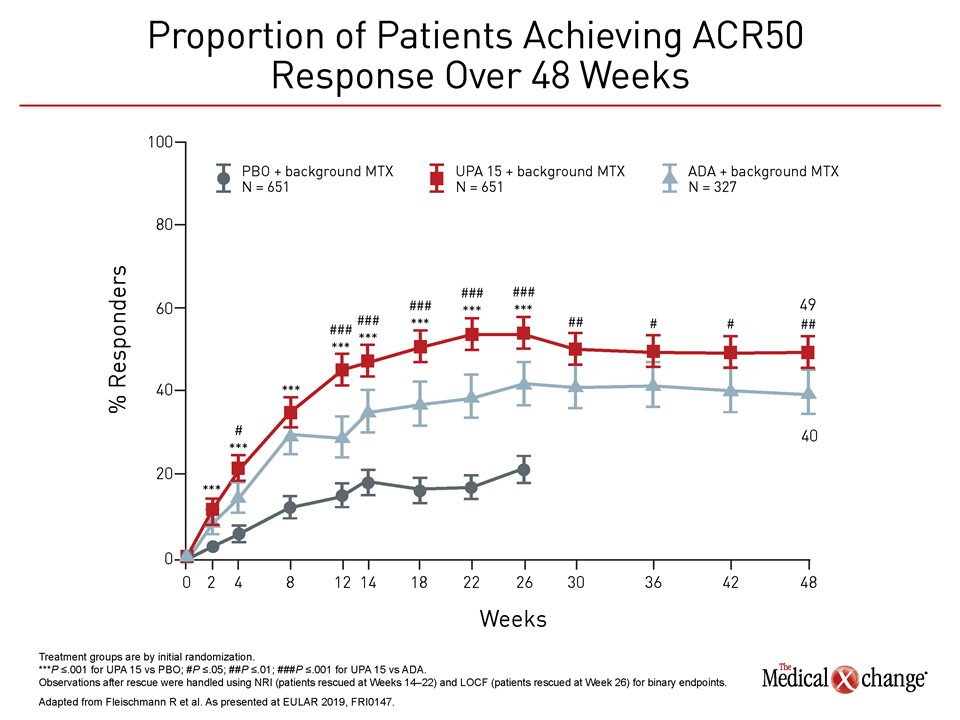
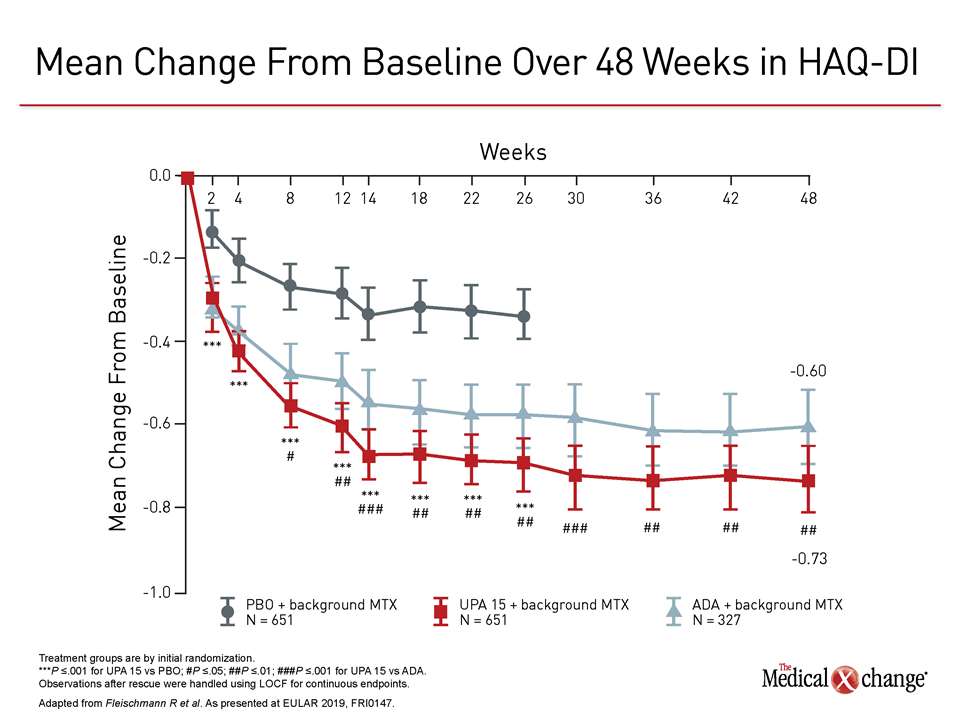
In both studies, objective differences in efficacy favored upadacitinib. In the trial comparing upadacitinib to adalimumab, called SELECT-COMPARE, newly-reported data at 48 weeks found the same relative advantage across multiple standardized measures for upadacitinib previously reported at 26 weeks. These included better disease control as measured with both the ACR50 and the Health Assessment Questionnaire Disability Index (HAQ-DI) assessment tools (Figure 1), (Figure 2).
“The relative advantage of upadacitinib over adalimumab included both clinical and functional responses that were seen early and persisted over the course of follow-up,” reported Dr. Roy M. Fleischmann, Clinical Professor of Medicine, University of Texas Southwestern Medical Center, Dallas.
“The relative advantage of upadacitinib over adalimumab included both clinical and functional responses that were seen early and persisted over the course of follow-up.”
In SELECT-COMPARE, 1,629 patients with an inadequate response to methotrexate were enrolled in a 2:1 ratio for the placebo and upadacitinib arms relative to the adalimumab arm. All patients remained on background methotrexate. At entry, more than half of patients were taking an oral glucocorticoid, but only 10% had prior exposure to a biologic disease modifying anti-rheumatoid arthritis drug (bDMARD). All enrolled patients were at high risk of progressive disease with a high-sensitivity C-reactive protein (hsCRP) level ≥5 mg/L and evidence of bone erosion in at least three joints.
Rescue Treatment Possible at 14, 18, and 22 Weeks
Upadacitinib was administered in a once daily oral dose of 15 mg. Adalimumab was injected in a dose of 40 mg every other week. Rescue treatment was permitted at weeks 14, 18, and 22 regardless of assigned therapy for failure to meet pre-specified criteria, particularly less than 20% improvement in the tender or swollen joint count. For placebo and adalimumab, the rescue was to upadacitinib. For upadacitinib, the rescue was to adalimumab. At week 26, all placebo patients were switched to upadacitinib.
As reported previously, 46.9% of the placebo patients and 23.5% of the adalimumab patients versus 19.2% of the upadacitinib patients had been rescued by week 26. By the end of the study, the proportion of patients requiring rescue was 48.6% in the adalimumab group but 38.7% in the upadacitinib group.
A surrogate measure of relative efficacy, the relative rescue rates were reflected in the outcome measures, according to Dr. Fleischmann. In addition to ACR50, he reported that there was a significant advantage at week 48 for ACR20, DAS28(CRP) ≤3.2, and DAS 28(CRP) ≤2.6.
Little Radiographic Progression at 48 Weeks
Relative to placebo, protection from radiographic structural damage was seen in both groups as measured with the modified Total Sharp Score (mTSS), joint space narrowing, and erosion score. The protection in the active treatment groups relative to placebo, first documented at 26 weeks, persisted to 48 weeks with little or no change in any measure between these time points. For example, the mean change in the mTSS score from baseline was 0.94 and 1.73 for the placebo groups at weeks 26 and 48, respectively. The scores at these time points, respectively, were 0.16 and 0.28 for patients on tofacitinib versus 0.19 and 0.39 for those treated with adalimumab.
Both agents were well tolerated with no significant differences in adverse events of interest. Typical of JAK inhibitors, the rate of herpes zoster infections was numerically higher on upadacitinib than adalimumab, but major adverse cardiovascular events (MACE) and venous thromboembolic events (VTE) were numerically lower.
Switch Analysis: Relative Response Rates
In a detailed analysis of relative responses when patients in SELECT-COMPARE trial were rescued for an inadequate response, patients benefited whether they were switched from adalimumab to upadacitinib or in the opposite direction. However, the data show a greater and more persistent benefit when patients on adalimumab were rescued by upadacitinib relative to the other way around.
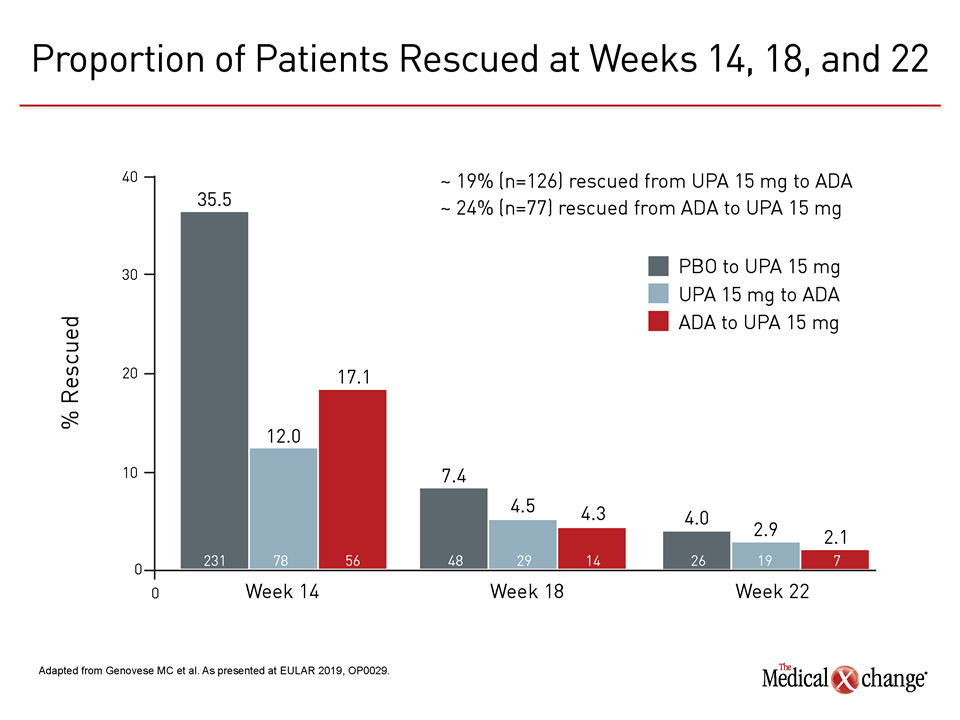
At 14 weeks, the first time point at which switches were permitted, 35% of placebo patients required rescue. Relative to placebo the rescue rate in the adalimumab group was 50% lower, but the rescue rate in the upadacitinib group was 30% lower than that of the adalimumab group. Rescue rates at subsequent time points were similar, but fewer upadacitinib patients overall required rescue (Figure 3).
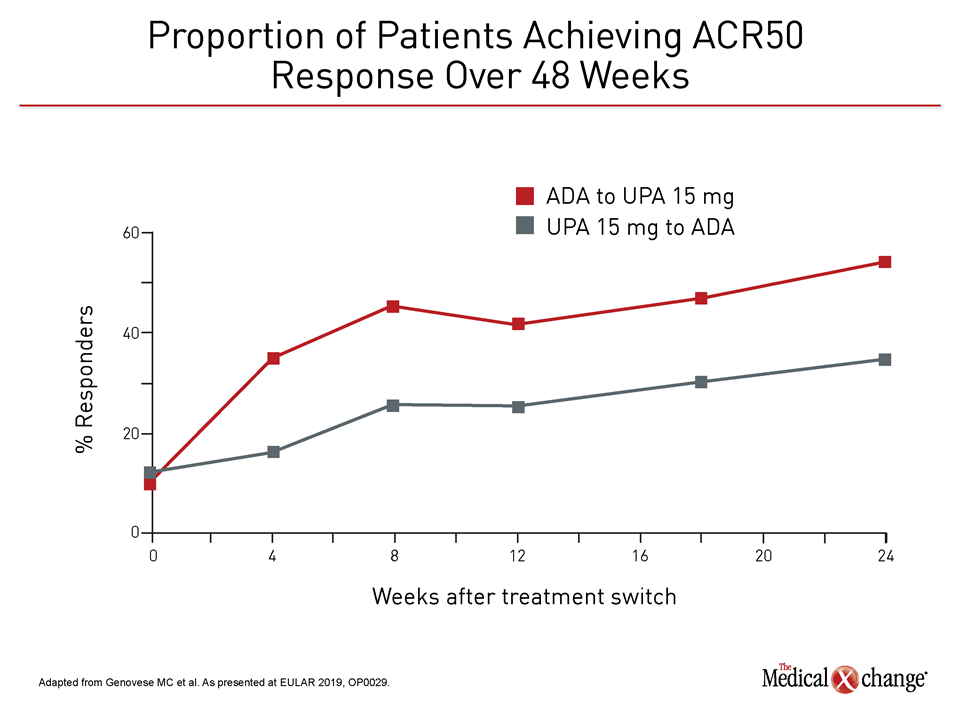
According to the principal investigator of this analysis, Dr. Mark Genovese, Director, Rheumatology Clinic, Division of Immunology and Rheumatology, Stanford University, Stanford, California, it is important to emphasize that there was substantial immediate and progressive benefit in both groups after the switch. However, the most recent data show that the efficacy advantages for those switched to upadacitinib relative to those switched to adalimumab, such as the proportion of patients with DAS28(CRP) <2.6, persisted at six months (Figure 4).
“The good news is that patients with an inadequate response to either upadacitinib or adalimumab benefited from switching to the alternate therapy.”
“The good news is that patients with an inadequate response to either upadacitinib or adalimumab benefited from switching to the alternate therapy,” Dr. Genovese reported. After the switch, “a substantial proportion of those switched reached treatment goals and realized meaningful improvement in clinical symptoms, HAQ-DI score, and pain.”
He further noted, “Despite an immediate switch without washout, there were no additional or unusual safety concerns observed in either treatment group.”
48-Week Data from SELECT MONOTHERAPY
Other long-term data from phase 3 trials in the upadacitinib pivotal trials program tell a similar story. Also presented at the 2019 EULAR Congress, new data from the SELECT-MONOTHERAPY trial found that the efficacy originally reported at the end of the 14-weeks remained robust at 48 weeks.
In this trial, patients on stable methotrexate therapy were randomized to continue on this treatment alone or switch to a 15 mg or 30 mg dose of upadacitinib. At the end of 14 weeks, the methotrexate patients were switched to one of the two upadacitinib arms pre-specified at enrollment. The addition of conventional DMARDs were permitted for those who did not achieve a Clinical Disease Activity Index (CDAI) <10 in the initial 14-week study period or during the blinded the long-term follow-up.
“At 48 weeks, there was persistent efficacy in the groups initiated on upadacitinib joined by similar responses at this time point from those switched from methotrexate at 14 weeks.”
“At 48 weeks, there was persistent efficacy in the groups initiated on upadacitinib as well as in those switched from methotrexate at 14 weeks,” reported Dr. Josef S. Smolen, Chairman, Department of Rheumatology, Vienna General Hospital, Austria.
This study, like others in the phase 3 program, supports the safety of upadacitinib. Pneumonia, occurring in <2% of patients, was the most commonly-reported serious adverse event. In an enrolled population of 532 patients initially assigned to upadacitinib, there were three VTES all of which occurred in patients with VTE risk factors. The safety analysis at 48 weeks did not reveal any new safety signals relative to the analysis at 14 weeks.
JAK Selectivity Drives New Agent Development
Newer JAK inhibitors are being pursued for the potential clinical advantage of greater relative selectivity on the JAK1 pathway, which is implicated in RA pathogenesis. Tofacitinib, although effective for RA, is a pan-JAK-STAT inhibitor. In theory, greater targeting of JAK1 relative to the other members of the JAK-STAT family, JAK2, JAK3, and Tyk2, has the potential to improve anti-inflammatory activity while avoiding off-target effects. In experimental studies, the selectivity of upadacitinib for JAK1 is 74-fold greater than that of JAK2 and 58-fold greater than that of JAK3. In comparison, baricitinib has about the same selectivity for JAK1 and JAK2 even though it is more selective for these than JAK3.
Head-to-head comparisons between JAK inhibitors with different relative JAK1 selectivity are awaited, but an indirect comparison between upadacitinib and tofacitinib was presented at the 2019 EULAR Congress. This analysis was conducted with a technique called a Matching-Adjusted Indirect Comparison (MAIC).
“This exploratory analysis compared the efficacy of these two JAK inhibitors after adjusting for differences across the trial populations,” explained Dr. Christopher Edwards, Chair of Clinical Rheumatology, University of Southampton NHS Foundation Trust, UK.
Matching Study Accounts for Patient Characteristics
In one of two MAIC analyses presented together, response rates among patients randomized to 15 mg upadacitinib in SELECT-MONOTHERAPY were compared to those of patients randomized to 5 mg tofacitinib in ORAL Standard, a pivotal phase 3 trial with tofacitinib that has been published. In the other, the 15 mg dose of upadacitinib and the 5 mg dose of tofacitinib were compared among patients participating in the SELECT-COMPARE and ORAL Strategy trials, respectively.
“On the basis of these MAIC analyses, 15 mg appears to provide improved outcomes at months three or six when compared to 5 mg tofacitinib.”
Matching included a wide variety of baseline variables, including biomarkers for inflammation, swollen joint count, and age. The drugs were compared on the basis of several outcomes, including ACR 20, ACR, 50, ACR 70 and DAS28(CRP).
In both analyses, response rates were significantly greater for upadacitinib relative to tofacitinib across several measures. In one of the two analyses, this included a significant advantage at six months of follow-up for proportion of patients in clinical remission defined by CDAI (P=0.038) and DAS28-ESR (P=0.003) with a trend for an advantage for clinical remission defined by DAS28(CRP) (P=0.057).
“The results with these two MAIC analyses suggest that treatment with 15 mg upadacitinib when used as monotherapy or in combination with methotrexate provides improved outcomes at months three or six when compared to 5 mg tofacitinib plus methotrexate,” Dr. Edwards reported. Direct, randomized and double-blind trials are essential to further explore this hypothesis, but Dr. Edwards said that these findings provide support for the hypothesis that JAK selectivity might be an important variable in eliciting anti-inflammatory responses.
Conclusion
As an oral targeted therapy, the JAK inhibitor tofacitinib has proven to be a valuable alternative to TNF inhibitors. Newer JAK inhibitors, such as upadacitinib and baricitinib, promise to expand treatment options within this class. While the hypothesis that greater JAK1 selectivity is relevant to the efficacy is still being explored, phase 3 data presented at the 2019 EULAR Congress demonstrate that JAK1 inhibitors offer substantial benefits in patients inadequately controlled on TNF inhibitors.