Expert Review
Proper Use of Anticoagulants for Stroke Prevention in Atrial Fibrillation
Chapter 1: Stroke Prevention in Atrial Fibrillation: The Guidelines
Gustavo Saposnik, MD MPH FRCPC
Associate Professor of Neurology
HSF Canada Career Scientist
Director, Outcomes Research & Decision Neuroscience Unit
St Michael’s Hospital, University of Toronto
Toronto, Ontario
In the setting of atrial fibrillation (AF), anticoagulation significantly reduces the risk of stroke. Yet, studies have shown repeatedly that AF patients have not received appropriate anticoagulation whether the goal is primary or secondary prevention. The problems have included failure to place AF patients on any anticoagulation, failure to employ a therapeutic dose, and failure of patients to adhere to their prescription. Understanding the reasons for the disappointing adherence to guidelines provides an opportunity to reverse an ongoing source of preventable mortality and morbidity. The goal of this program will be to emphasize the risk of stroke in patients with AF and clarify the evidence-based strategies for the proper management of AF patients whether preventing a first stroke or recurrent strokes. With greater adherence to proper use of anticoagulation, a substantial reduction in preventable morbidity and stroke-related death can be anticipated.
Epidemiology of Stroke in Atrial Fibrillation
Atrial fibrillation (AF) is an important but readily modifiable risk factor for stroke.1 Patients with AF have a five-times greater risk of stroke than those without AF.2 Of ischemic strokes, which account for approximately 80% of all strokes,3 20% to 30% are attributed to AF, but the proportion grows in aging patients, exceeding 35% in those older than 80 years of age.4 In Canada, where stroke is the third most common cause of death and tenth largest cause of disability,5,6 strategies to reduce risk of stroke in AF patients represents a major target of avoidable morbidity and mortality. In AF patients who have experienced an initial stroke, the age-adjusted risk for a recurrent stroke in the absence of anticoagulation is increased by more than two-fold.7
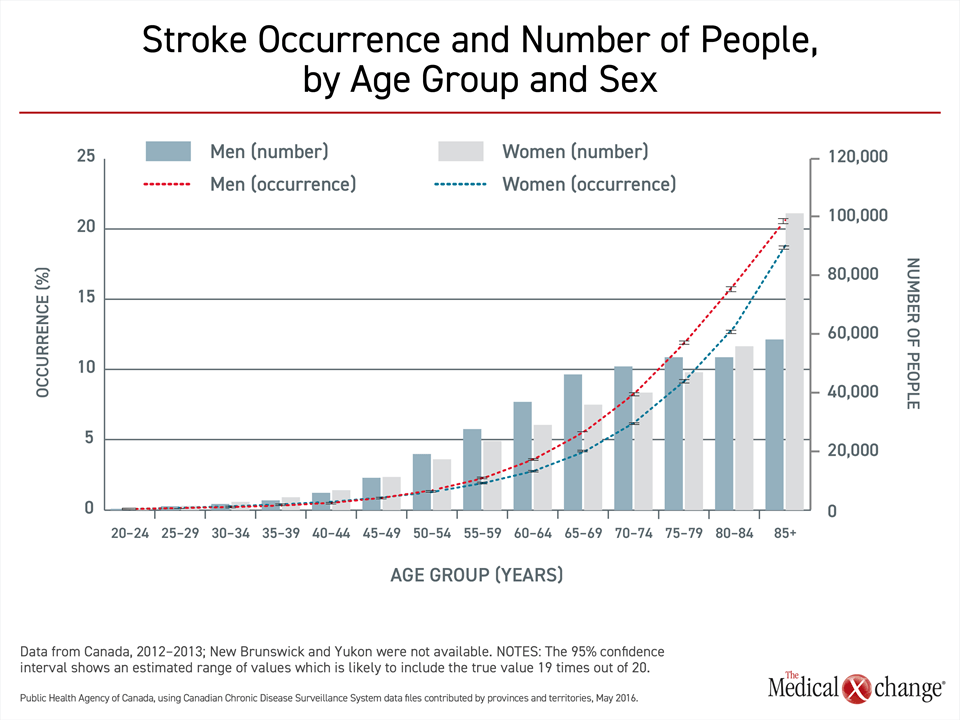
Whether strokes are ischemic or hemorrhagic, risk increases with age.3 Calculated from the age of 25, the lifetime risk of stroke is 25%,8 but events track with the prevalence of modifiable risk factors for vascular disease, including hypertension, diabetes mellitus, dyslipidemia, and obesity.3 As a result, stroke incidence in Canada, as elsewhere, remains low until late middle age when the consequences of poor lifestyle choices, such as sedentary behavior and an unhealthy diet, contribute to vascular diseases, including stroke (Fig. 1).
In Canada, about 10% of adults aged 65 years or older have experienced a stroke, and more than 400,000 individuals are living following a stroke.9 The stroke incidence is modestly higher in men than women, but women represent a greater proportion of survivors 80 years of age or older. From 2003 to 2012, there was a decline in the incidence and mortality attributed to stroke in Canada, due possibly to better and more prompt treatments, but the absolute number of stroke survivors is rising due both to population growth and the aging of the Canadian population.9
AF, the most common type of cardiac arrhythmia, is also age-related. With a lifetime risk of approximately 25%, the prevalence of AF doubles with every decade of life.10 Due to the increased life expectancy and demographic shift that is increasing the proportion of individuals aged 65 or older in Canada and many other countries, an epidemic of AF that is already underway is expected to persist for the next two decades.11 In addition to age, many risk factors for ischemic stroke, including hypertension, obesity, diabetes mellitus, and coronary artery disease, are shared with AF.12 By itself, AF is associated with a two-fold increase in all-cause mortality in women and a 50% increase in men.13 Stroke is only one contributor. Nearly one-third of patients have left ventricular dysfunction by the time they develop AF.12 Disseminated vascular disease is implicated in the high rates of deaths due to myocardial infarction and other heart-related causes in patients with AF as well as in the subclinical ischemia that is now understood to be the cause of neurological deficits in AF patients even in the absence of stroke.14,15
Furthermore, stroke patients with AF are more likely to have a higher disability and mortality. For example, a study from Ontario, Canada comprising over 12,000 patients with an acute ischemic stroke showed 30% lower likelihood of being independent at 30 days among patients with AF compared to those without AF.16 In this study, stroke patients with AF also had higher risk of death at 30 days (22.3% versus 10.2%; P<0.0001), 1 year (37.1% versus 19.5%; P<0.0001) and death or disability at discharge (69.7% versus 54.7%; P<0.0001) compared with non-AF patients. In addition, patients with AF were less likely to recover from intravenous thrombolysis compared to those without AF. Other studies have found similar results.4,17 In patients with a first stroke, a new diagnosis of AF is associated with an increased risk of recurrent stroke even relative to patients with known AF.18
As a consequence, both primary and secondary stroke prevention among patients with AF are critical. In patients with AF, there is a near universal benefit from stroke prophylaxis with anticoagulation therapy, according to multiple evidence-based guidelines.12,19-21 This is due to very high rates of death and disability that preserve a favourable benefit-to-risk ratio for primary or secondary prevention in the vast majority of individuals with AF. Relative to individuals who develop stroke in the absence of AF, AF patients are significantly more likely to be chronically disabled following stroke (P<0.0005) and nearly twice as likely to die within six months (P<0.001).4,17 AF patients with even minor strokes or transient ischemic attacks (TIAs) are at increased risk of cardiovascular events as well as recurrent strokes.21 Consequently, risk classification is critical for stroke prevention among patients with AF. For example, a previous study on nearly 100 opinion leaders revealed risk classification errors in 50% of simulated AF scenarios and therapeutic inertia, defined as lack of initiation of OAC, in 60%.22
The Solution: Managing Stroke Risk in Patients with AF
Each of the major guidelines for the management of AF, including those written for Canada,19 the United States,20 and Europe,12 recommend oral anticoagulation for primary stroke prevention, identifying candidates with risk scoring systems. In Canada, guidelines for secondary prevention of stroke emphasize the importance of screening for AF and implementing anticoagulation as well as vascular risk modification in such patients.21
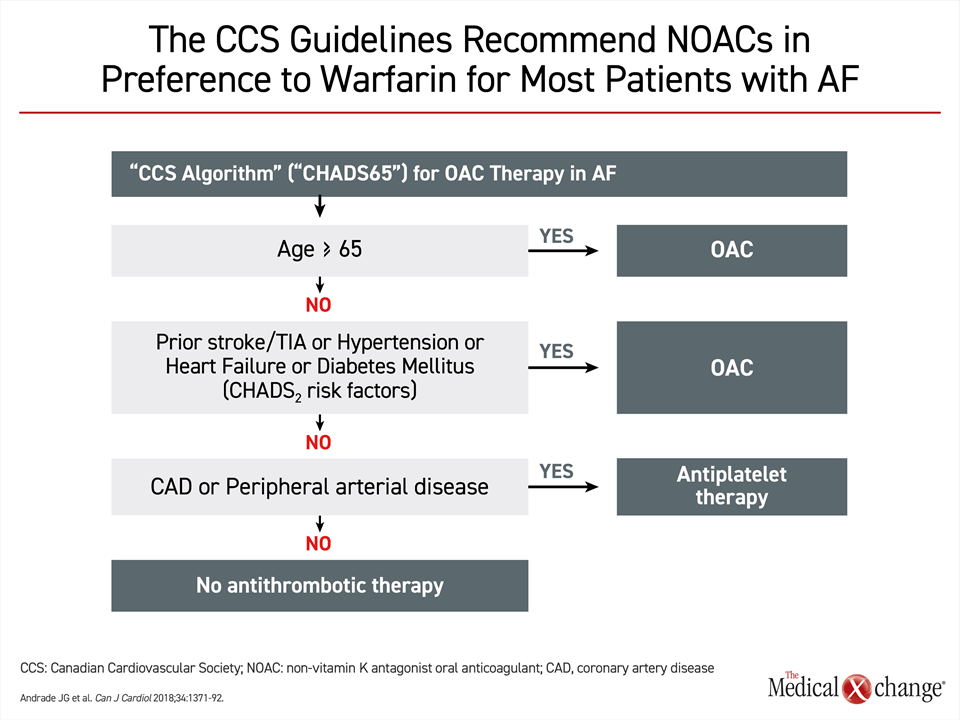
The European and U.S. guidelines both identify candidates for anticoagulation with CHA2DS2-VASc (Congestive heart failure, Hypertension, Age, Diabetes, Stroke, Vascular Disease, Age and Sex), which is an updated version of CHADS2, which did not consider vascular disease or gender.23 The Canadian Cardiovascular Society (CCS) guidelines employ CHADS-65, a simplified version of CHADS2.19 According to the CHADS-65 algorithm, oral anticoagulation should be routinely considered in any AF patients more than 65 years of age (Fig. 2). It is also recommended for younger patients with any of the risk factors identified in CHADS2, which includes prior stroke, hypertension, heart failure, or diabetes mellitus. Due to the frequency with which AF occurs in patients over the age of 65 years or in those with common cardiovascular risk factors, such as hypertension, most patients with AF are likely to be candidates for non-vitamin K antagonist oral anticoagulants (NOACs) for primary stroke prevention.
Although based on CHA2DS2-VASc, the European guidelines for primary stroke prevention are similar.12 Oral anticoagulation is recommended for any man with a score of one or greater and any woman with a score of two or greater. A score of one is obtained for anyone of 65 years or greater and anyone with a history of vascular disease, defined as history of myocardial infarction, peripheral artery disease, or aortic plaque. An age of 75 years or older is given a score of two. The U.S. guidelines call for two risk factors in men and three in women irrespective of age,20 but, again, exemptions on this basis would be expected to be limited among patients in the age range where AF typically occurs.
The Canadian Stroke Best Practice Recommendations (CSBPR) for secondary stroke prevention calls for routine screening for AF.21 In those with AF, the same protection from the high rates of disability and mortality are anticipated from preventing a second stroke as from preventing the first.24,25 In long-term management, NOACs are the preferred treatment option for most patients with AF and recurrent stroke. Patients with a mechanical heart valve are an exception. In those taking warfarin, dosing within target international normalized ratio (INR) is essential for optimizing benefit. These climb from a range of 2.0 to 3.0 in the absence of a mechanical heart valve to 2.5 to 3.5 in those with a valve. For those taking NOACs, patients should be informed of the danger of missed doses and monitored routinely for adherence. To adjust NOAC doses appropriately, renal function should be assessed annually or more often in those at risk of altered renal function.
All of the major guidelines now recommend NOACs over the vitamin K antagonist warfarin, which had once been a standard.12,19,20 There are four agents in this class approved in Canada. Although warfarin remains an acceptable alternative in each of the guidelines, the NOACs are preferred on the basis of clinical trials showing similar or greater efficacy with similar or lower rates of the intracranial haemorrhage, the most feared type of bleeding. In addition, NOACs are easier to use. Unlike warfarin, they do not require INR monitoring and dose adjustments to ensure adequate anticoagulant effect.
In general, AF patients at increased or high risk of bleeding are not contraindicated for oral anticoagulation. The European guidelines, for example, recommend identifying and treating modifiable risk factors for bleeding but conclude that oral anticoagulation should not generally be withheld due to an elevated bleeding risk.12 The CCS guidelines for prevention of stroke in patients with AF also acknowledge bleeding risk factors without specific limitations on anticoagulant prophylaxis.19
Pivotal Trials: NOACs as Standard for Stroke Prevention
Warfarin is highly effective for stroke prevention in AF patients,26 but the preference for NOACs in current guidelines is evidence-based. NOACs are not interchangeable for important characteristics, such as safety in patients with compromised kidney function, but pivotal trials with each of these agents showed each to be at least as effective and safe as warfarin. The four NOACs available in Canada are the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban, and dabigatran, a direct thrombin inhibitor.
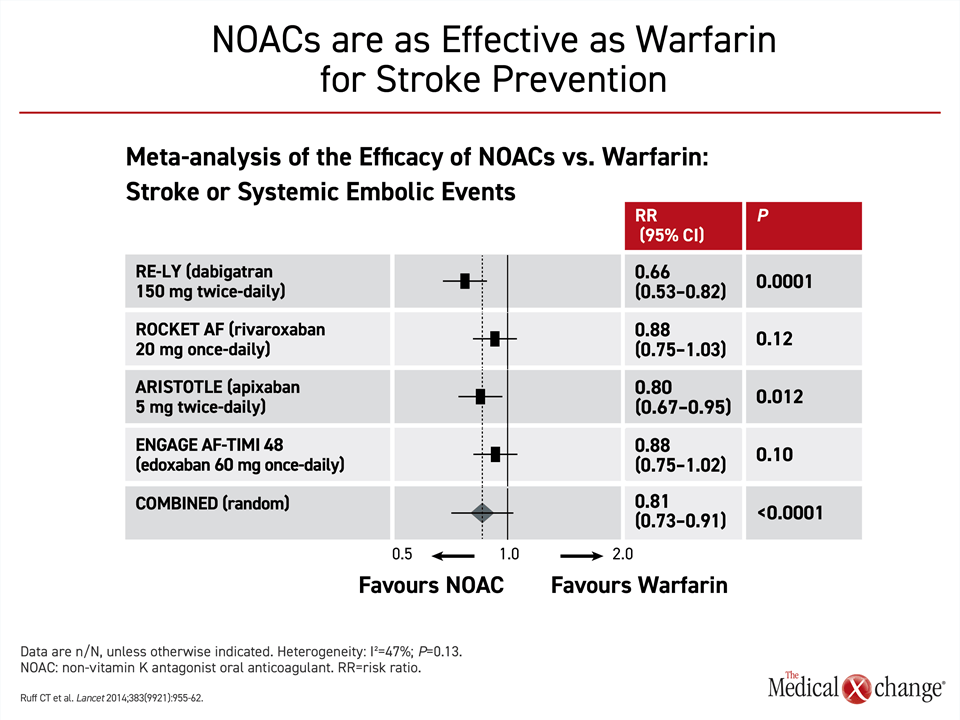
The pivotal NOAC trials had similar designs. In each, the NOAC was tested for non-inferiority to warfarin for the primary outcome of stroke or embolism. All NOACs were associated with at least a numerically lower risk of the primary outcome relative to warfarin with the difference reaching significance in the RE-LY trial with dabigatran. In a meta-analysis of these trials, the 19% reduction for NOACs versus warfarin was highly significant (P<0.0001)27 (Fig. 3).
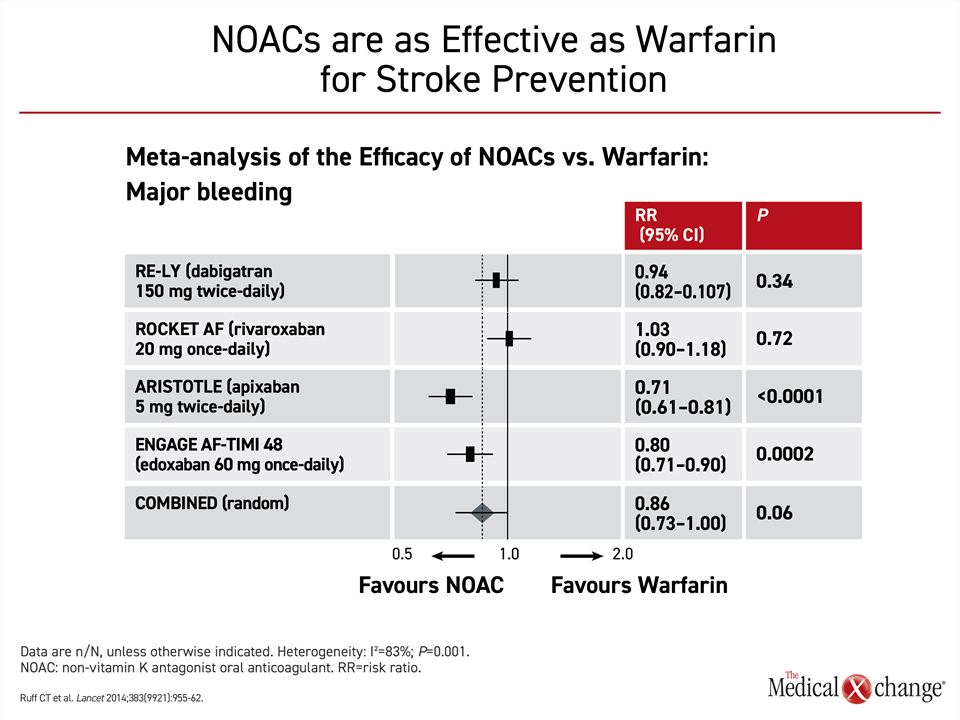
When compared for bleeding events, the pivotal trials indicated that risk of bleeding was no greater on NOACs than warfarin. In two of the trials, ARISTOLE with apixaban (P<0.0001) and ENGAGE AF-TIMI 48 trial with edoxaban (P=0.0002), there was a significant advantage for the NOAC over warfarin. In the meta-analysis of these pivotal NOAC trials, the reduction in the bleeding risk for NOACs approached statistical significance (P=0.06) (Fig. 4).
In ROCKET-AF,28 rivaroxaban was non-inferior to warfarin for the primary endpoint on an intention-to-treat analysis but statistically superior on the per-protocol analysis. Rivaroxaban was associated with an increase in some non-fatal bleeding events, but a significant reduction in hemorrhagic stroke and intracranial bleeding. No mortality advantage was observed.
In the ARISTOTLE trial,29 apixaban was associated with a significant 21% reduction in the primary outcome, a 31% reduction in major bleeding, and an 11% reduction in all-cause mortality. In a further breakdown of secondary endpoints, apixaban was associated with a significant reduction in hemorrhagic stroke and intracranial bleeding relative to warfarin, but ischemic stroke was not significantly reduced.
In the ENGAGE AF-TIMI 48 trial,30 the 60 mg dose of edoxaban was associated with a 21% reduction in the primary endpoint relative to warfarin (P<0.001) along with a 20% reduction in risk of major bleeding (P<0.001). The 60 mg dose of edoxaban, which is the standard, was also associated with a relative 14% reduction (P=0.013) in cardiovascular death and 10% reduction (P=0.02) in a composite secondary endpoint of stroke, systemic embolic event, or death.
In the RE-LY trial,31 the 150 mg dose of dabigatran was associated with a 35% reduction in the primary endpoint relative to warfarin. Ischemic stroke was reduced by 24% and vascular mortality was reduced by 15%. A 12% reduction in death from any cause narrowly missed statistical significance (P=0.051). The 150 mg dose of dabigatran was associated with increased risk of gastrointestinal bleeding but not in major bleeding. It was associated with a 60% reduction in intracranial bleeding.
It is not possible to compare NOACs across trials, but the NOACs are collectively considered to have a favorable benefit-to-risk ratio relative to warfarin. In a meta-analysis of the more than 70,000 patients enrolled in these four trials, NOACs were associated with a 19% reduction (P<0.0001) in stroke or systemic embolic events and a 10% reduction (P=0.0003) in all-cause mortality.27 The meta-analysis also associated NOACs with a 51% reduction (P<0.0001) in intracranial hemorrhage.
In this meta-analysis, gastrointestinal bleeding was 25% higher (P=0.04) on NOACs relative to warfarin, but there was significant heterogeneity (P=0.001) among the NOACs for the category of major bleeding.27 The combined data associated NOACs with a 14% reduction in this outcome relative to warfarin, which did not reach significance, but the 29% reduction in risk of bleeding associated with 5 mg apixaban (P<0.0001) and the 20% reduction (P=0.0002) associated with edoxaban were both significant. The 6% reduction associated with 20 mg rivaroxaban was not, and major bleeding was increased by a non-significant 3% for 150 mg of dabigatran relative to warfarin.
Despite variability in the efficacy and safety outcomes, the NOACs as a group offer a favourable risk-to-benefit ratio relative to warfarin, according to the authors of this meta-analysis.27 In addition, the favourable efficacy and safety was characterized as consistent across a wide range of patients. Differences between NOACs might be relevant in some patient groups, such as the elderly,32 but these data support NOACs as a guideline-directed preferred therapy.10,12,20
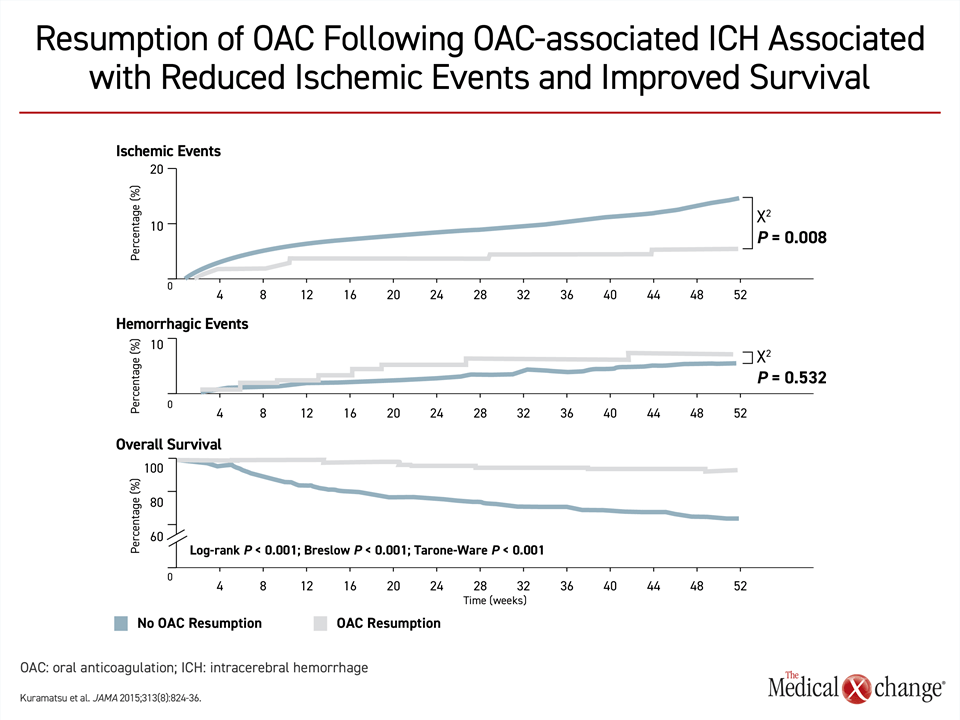
The relative advantages of NOACs over warfarin extend to patients with recurrent stroke, according to the CSBPR guidelines,21 but the timing of resumption of anticoagulation remains controversial. Although a mortality benefit from restoring anticoagulation has been observed even in stroke patients who have had a prior intracerebral hemorrhage (ICH)33 (Fig. 5), there is concern that resumption too soon after a first ischemic stroke might increase risk of ICH whereas resumption too late might leave patients vulnerable for recurrent stroke. The data from published studies, most of which are small or retrospective, have been inconsistent.34 Although the European Society of Cardiology advocates a 1-3-6-12 rule of thumb,35 which calls for resumption of anticoagulation one day after a transient ischemic attack, three days after a non-disabling stroke, six days after a moderate stroke, and 12 days after a major stroke, guidelines in the United Kingdom and Germany conclude that there is insufficient data to judge safety and efficacy at a start day anytime within 14 days of a stroke.34
Ongoing Trials Hope to Determine Optimal Timing for Treatment Initiation
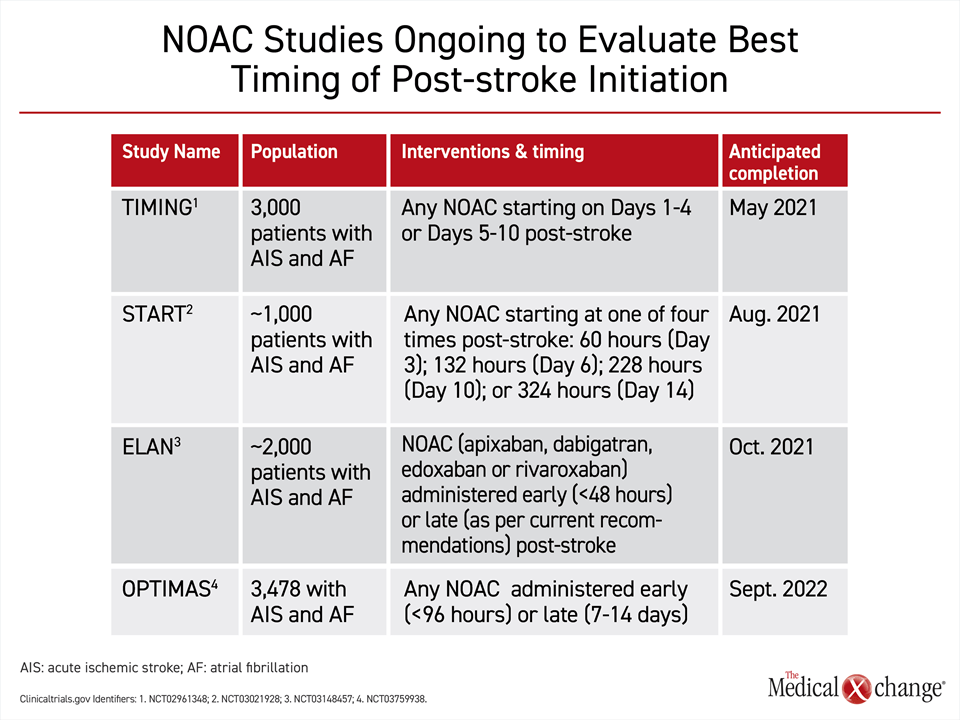
The question of the optimal timing for resumption or new start of an oral anticoagulant might be settled by four large randomized multicenter trials that are now ongoing (Fig. 6). Ranging in size from 1000 to nearly 4000 patients, three of the trials, TIMING, START, and ELAN, are scheduled for completion in 2021. A fourth trial will be completed in 2022. Each is comparing an early to some later resumption of a NOAC after an ischemic stroke in AF patients. Designs differ, but an early start is generally defined as within four days. The delayed starts range from five days to more than 14 days after stroke. In each of the trials, patients are permitted to take any of the four currently approved NOACs. These four trials hope to provide further insight into whether early NOAC treatment has the same degree of safety and efficacy as late treatment initiation. Trial results in favour of early treatment would likely impact patient convenience via early hospital discharge as well as clinical practice in regards to improved compliance and continuation of anticoagulant medication started in hospital.
Oral Anticoagulation in the Emergency Department Upon AF Diagnosis
Upon diagnosis of AF in an emergency department, new evidence shows that patients are more likely to fill a prescription for oral anticoagulation if it is provided prior to discharge.36 In this cohort study involving 15 Canadian hospitals, the absolute risk of not filling a prescription for oral anticoagulation at six months was increased by 30.6%. At 12 months, it was increased by 23.2%. The numbers needed to treat at these time intervals were three and four, respectively. The disparities in adherence point to one source for the therapeutic gap in stroke prevention.
Summary
Oral anticoagulation is a proven strategy for reducing the high clinical toll imposed by primary and secondary stroke in AF patients. According to major guidelines, including those issued in Canada, the evidence base for major risk reductions with oral anticoagulation in general and NOACs specifically is compelling. The guidelines for both primary and secondary prevention are simple. All patients without a clear contraindication should receive a NOAC, typically administered in standard doses. In the CCS guidelines, only those under the age of 65 years without history of heart failure, hypertension, diabetes mellitus, prior thromboembolism, or cerebrovascular attack are exempted from primary stroke prevention.19 For secondary stroke prevention, the CSBPR indicate that all AF patients should be considered candidates for oral anticoagulation. Adherence to current guidelines offers an opportunity to reduce an important source of morbidity and mortality in Canada.
References
- Wolf PA, Dawber TR, Thomas HE, Jr., Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology 1978;28:973-7.
- Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82:2N-9N.
- Boehme AK, Esenwa C, Elkind MS. Stroke Risk Factors, Genetics, and Prevention. Circ Res 2017;120:472-95.
- Reiffel JA. Atrial fibrillation and stroke: epidemiology. Am J Med 2014;127:e15-6.
- StatisticsCanada. Leading causes of death, total population, by age group and sex, Canada. http://www5statcangcca/cansim/a05?lang=eng&id=1020561 2017.
- DALYs GBD, Collaborators H. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1603-58.
- Penado S, Cano M, Acha O, Hernandez JL, Riancho JA. Atrial fibrillation as a risk factor for stroke recurrence. Am J Med 2003;114:206-10.
- Collaborators GBDLRoS, Feigin VL, Nguyen G, et al. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N Engl J Med 2018;379:2429-37.
- CCDSS. Stroke in Canada: highlights from Canadian Chroni Disease Surveillance System (CCDSS). https://wwwcanadaca/en/public-health/services/publications/diseases-conditions/stroke-canada-fact-sheethtml 2019:Accessed June 1, 2020.
- Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453-68.
- Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol 2017;14:195-203.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893-962.
- Andersson T, Magnuson A, Bryngelsson IL, et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995-2008: a Swedish nationwide long-term case-control study. Eur Heart J 2013;34:1061-7.
- Hahne K, Monnig G, Samol A. Atrial fibrillation and silent stroke: links, risks, and challenges. Vasc Health Risk Manag 2016;12:65-74.
- Gomez-Outes A, Suarez-Gea ML, Garcia-Pinilla JM. Causes of death in atrial fibrillation: Challenges and opportunities. Trends Cardiovasc Med 2017;27:494-503.
- Saposnik G, Gladstone D, Raptis R, et al. Atrial fibrillation in ischemic stroke: predicting response to thrombolysis and clinical outcomes. Stroke 2013;44:99-104.
- Gattringer T, Posekany A, Niederkorn K, et al. Predicting Early Mortality of Acute Ischemic Stroke. Stroke 2019;50:349-56.
- Kamel H, Johnson DR, Hegde M, et al. Detection of atrial fibrillation after stroke and the risk of recurrent stroke. J Stroke Cerebrovasc Dis 2012;21:726-31.
- Andrade JG, Verma A, Mitchell LB, et al. 2018 Focused Update of the Canadian Cardiovascular Society Guidelines for the Management of Atrial Fibrillation. Can J Cardiol 2018;34:1371-92.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125-e51.
- Wein T, Lindsay MP, Cote R, et al. Canadian stroke best practice recommendations: Secondary prevention of stroke, sixth edition practice guidelines, update 2017. Int J Stroke 2018;13:420-43.
- Sposato LA, Stirling D, Saposnik G. Therapeutic Decisions in Atrial Fibrillation for Stroke Prevention: The Role of Aversion to Ambiguity and Physicians’ Risk Preferences. J Stroke Cerebrovasc Dis 2018;27:2088-95.
- Chen JY, Zhang AD, Lu HY, Guo J, Wang FF, Li ZC. CHADS2 versus CHA2DS2-VASc score in assessing the stroke and thromboembolism risk stratification in patients with atrial fibrillation: a systematic review and meta-analysis. J Geriatr Cardiol 2013;10:258-66.
- Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MS. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology 2006;66:641-6.
- Aarnio K, Haapaniemi E, Melkas S, Kaste M, Tatlisumak T, Putaala J. Long-term mortality after first-ever and recurrent stroke in young adults. Stroke 2014;45:2670-6.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-67.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955-62.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51.
- Kato ET, Goto S, Giugliano RP. Overview of oral antithrombotic treatment in elderly patients with atrial fibrillation. Ageing Res Rev 2019;49:115-24.
- Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 2015;313:824-36.
- Seiffge DJ, Werring DJ, Paciaroni M, et al. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol 2019;18:117-26.
- Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013;34:2094-106.
- Atzema CL, Jackevicius CA, Chong A, et al. Prescribing of oral anticoagulants in the emergency department and subsequent long-term use by older adults with atrial fibrillation. CMAJ 2019;191:E1345-E54.
Chapter 1: Stroke Prevention in Atrial Fibrillation: The Guidelines
In the setting of atrial fibrillation (AF), anticoagulation significantly reduces the risk of stroke. Yet, studies have shown repeatedly that AF patients have not received appropriate anticoagulation whether the goal is primary or secondary prevention. The problems have included failure to place AF patients on any anticoagulation, failure to employ a therapeutic dose, and failure of patients to adhere to their prescription. Understanding the reasons for the disappointing adherence to guidelines provides an opportunity to reverse an ongoing source of preventable mortality and morbidity. The goal of this program will be to emphasize the risk of stroke in patients with AF and clarify the evidence-based strategies for the proper management of AF patients whether preventing a first stroke or recurrent strokes. With greater adherence to proper use of anticoagulation, a substantial reduction in preventable morbidity and stroke-related death can be anticipated.
Show review