Expert Review
The Aging HIV Patient
Chapter 4: Neurocognitive Deficit
Bruce James Brew, MD
University of New South Wales, Sydney, Australia
Cognitive decline is an insidious and frequently progressive complication of human immunodeficiency virus (HIV) infection. While the introduction of highly active antiretroviral therapy (HAART) largely eliminated the dementia associated with end-stage acquired immunodeficiency syndrome (AIDS), cognitive impairment remains a common complication of HIV even in those with well controlled viremia. The incidence and the severity of HIV-associated neurocognitive disorders (HAND) are rising with an aging HIV population – a potential consequence of both increased susceptibility in older individuals and progressive impairment with longer duration of infection. The clinical challenges posed by this complication are substantial. The relative ability of specific antiretroviral therapies to cross the blood brain barrier to reduce central nervous system (CNS) viral load, and the specific, perhaps genetic, susceptibility of individual patients are among factors that may explain the variable risk of cognitive impairment. In addition, HIV-independent variables may also be important in some individuals. Clinical sensitivity to changes in cognitive function through periodic assessments is appropriate in the context of strategies to understand and prevent HAND.
Neurocognitive Loss: Epidemiology
Prior to the introduction of highly active antiretroviral therapy (HAART), dementia was a common acquired immunodeficiency syndrome (AIDS)-defining complication of human immunodeficiency virus (HIV) infection, occurring in at least 15% of patients by the time of death (1). In this pre-HAART era, the risk of AIDS-dementia complex (ADC) increased with diminishing immune function and appeared to be a direct effect on uncontrolled HIV infection (2). In the post-HAART era, ADC has been joined by HIV-associated neurocognitive disorders (HAND), the inclusive term that describes the spectrum of HIV-associated decrements in neurologic function from ADC to asymptomatic neurocognitive impairment (3). Although HAART has significantly reduced the risk of dementia, the rates of HAND appear to be unchanged after adjusting for age, education, gender, race, and CD4 count (4). The evidence of progressive cognitive deficits observed in patients even with well controlled viremia suggests an active intracerebral process that persists even when clinical signs of infection have otherwise been eliminated (5). In a recently completed cross-sectional study, dementia was rare, but HAND was common with 52% of patients having some degree of neuropsychological impairment (6). Specifically, 33% had asymptomatic cognitive impairment, 12% had mild but symptomatic cognitive impairment, and 2% had dementia, while the remaining had other causes of neuropsychological deficit.
Cognitive loss progresses over time even in patients with HIV that is well controlled on the basis of plasma viremia levels.
Cognitive loss progresses over time even in patients with HIV that is well controlled on the basis of plasma viremia levels. In one study that evaluated HIV-infected patients with undetectable viremia, up to one third demonstrated a decline in function over 27 months of follow-up (7). Treatable causes of cognitive loss were ruled out. Age and duration of HIV infection are likely to be risk factors for cognitive loss. In a prospective study comparing one cohort of patients older than age 50 years to a second cohort between the ages of 20 and 39 years, the incidence of cognitive loss was twice as great in the older group (25% vs. 13%; P=0.041) (8). In a large cohort study that looked at multiple risk factors for cognitive impairment in the post-HAART era, both older age at the time of seroconversion and the duration of infection were independent predictors (9). The potential for an interaction between age, duration of infection, and HIV-independent risk factors for cognitive dysfunction, such as cerebrovascular disease, are potentially important in the aging HIV population (10). Although a substantial proportion of HIV-infected patients have not developed detectable cognitive impairment even after long duration of infection, suggesting that some genetic susceptibility factors may be important (11), there are numerous other variables that appear to influence risk in individuals whose viremia is otherwise well controlled. For example, one study found rates of global cognitive impairment to be almost 50% higher (63% vs. 43%) in patients co-infected with hepatitis C when compared to those with HIV alone (12). Biomarkers to predict cognitive dysfunction in patients with HIV are actively being pursued (13). Although it is not clear whether cognitive loss shortens survival, except in advanced HIV (14), high rates of cognitive loss in aging patients with HIV threaten a major public health crisis.
Pathogenesis
It is likely that HIV crosses the blood-brain barrier into the CNS through a subset of infected monocytes establishing a CNS reservoir of HIV capable of neurotoxicity sometime after seroconversion.
The pathophysiology of HAND is driven by neurotoxic HIV viral proteins generated by infected cells, which include astrocytes and microglia as well as macrophages, and by neurotoxic cytokines, chemokines, and other mediators generated by pro-inflammatory signalling from non-infected cells in the CNS (15). It is likely that HIV crosses the blood-brain barrier into the CNS through a subset of infected monocytes establishing a CNS reservoir of HIV capable of neurotoxicity sometime after seroconversion, possibly early (16). Over subsequent years of infection, HIV evolves in the CNS occurs independently of the virus in the periphery (17). There are several mechanisms for neuronal and neural damage encompassing synapto-dendritic injury but oxidative stress including excitotoxicity is the most important (18, 19)Autopsy studies reveal dominantly subcortical mononuclear cell infiltrates with microglial nodules, multinucleated giant cells, and gliosis (20). In HAART-treated patients, there are fewer infiltrates and in some there are overlapping features with Alzheimer’s disease, including elevated amyloid deposition (21). Cognitive function can be stratified into a wide range of measures, such as speed of information processing, attention, memory storage, and memory access, that involve different processes in different areas of the brain (22). Due to differences in the types of cognitive losses associated with specific pathologies, a variety of measures are used to document different types of change. In one study, diminished motor skills and verbal fluency were found to be common examples of cognitive loss in the pre-HAART era, whereas loss of memory and executive function have been more common in aging patients with well controlled viremia (23). Other studies corroborate the types of cognitive deficits typical in aging adults, such as loss of memory storage and retrieval (23-25), suggesting that HIV-associated cognitive loss in the HAART era overlays a parallel pathology associated with aging, to produce a compounding of effects (26). Importantly, the cerebrospinal fluid (CSF) penetration of antiretroviral agents differsmarkedly even within drug classes, such as protease inhibitors (PIs) or nucleoside reverse transcriptase inhibitors (NRTIs) (26). In a ranking system based on several variables, including CSF levels, most PIs had low penetration on the three-level stratification, but ritonavir-boosted indinavir, amprenavir, lopinavir and darunavir demonstrated high penetration (27-28). In the NRTI class, tenofovir and didanosine had the lowest ranked penetration, while abacavir and zidovudine were among those with the highest penetration. The premise that reduced viral levels in the CSF will the reduce production of neurotoxic proteins to prevent or slow cognitive loss is compelling, but not yet well established in prospective trials. Antiretroviral regimens containing agents predicted to have poor penetration are associated with higher CSF levels of HIV RNA than regimens predicted to have good penetration (27), but few prospective studies have demonstrated a difference in cognitive outcome from high penetration agents. In an twelve-week trial, abacavir was not more effective than placebo in improving cognitive function when either was added to a stable background regimen, but this trial was of relatively short duration and only one agent, as opposed to several, was added to the regimen (29). Overall, the data associating poorer CSF penetration with increased HIV replication in the CSF do support further definitive randomized clinical trials comparing antiretroviral regimens with variable effects on CSF for impact on neurocognitive outcomes (27, 30). HIV-related frailty is a relatively recently reported phenomenon that may reflect accelerated aging across physiologic systems, including CNS function (31). In a study comparing individuals initiating antiretroviral therapy over the age of 50 years, the risk of cardiovascular, metabolic or neurologic disorders was more than six times greater (P<0.0001) when compared to a younger cohort even though the antiretroviral regimens were similarly well tolerated (32). This may be a related independent mechanism of accelerated CNS pathology in aging HIV-infected individuals.
Diagnosis and Monitoring
In the post-HAART era, early and subtle signs of cognitive loss do not necessarily signal imminent and clinically significant morbidity but represent an opportunity for early intervention.
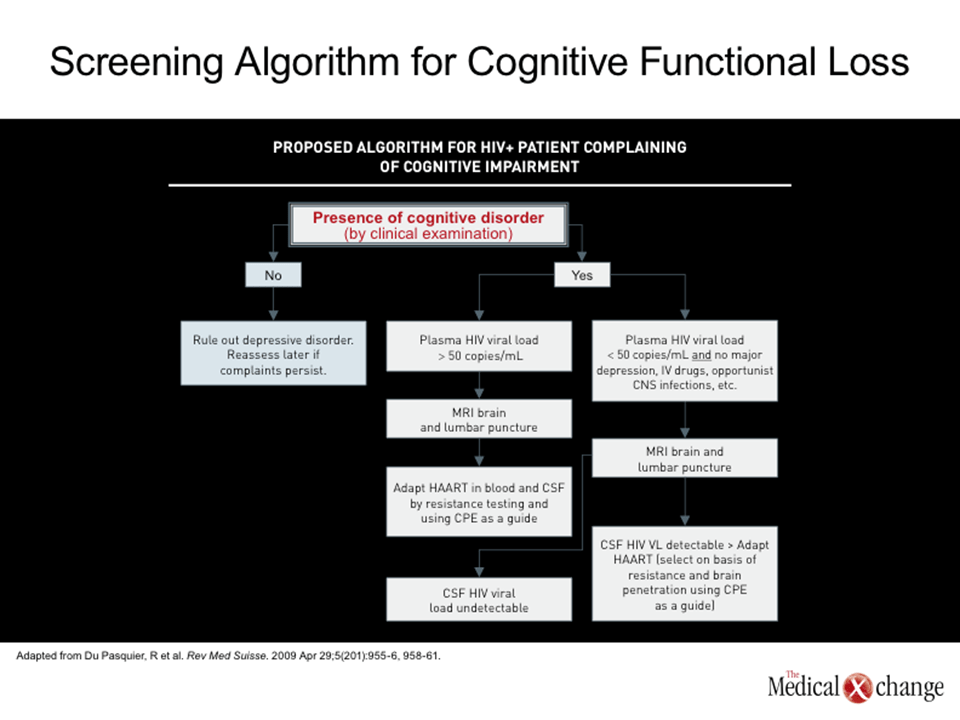
Based on the high rates of cognitive impairment in individuals with HIV, it is appropriate to perform periodic cognitive testing in all individuals with this infection. Even in relatively young patients, baseline measures permit subsequent changes in cognitive function to be documented. The American Academy of Neurology (AAN) first defined HIV-associated cognitive impairment in 1991 (33), but the significance of cognitive loss in the post-HAART era has evolved. Before HIV viremia could be controlled, the onset of dementia was frequently a signal of advanced immunologic deterioration that predicted the end stages of disease. In the post-HAART era, early and subtle signs of cognitive loss do not necessarily signal imminent and clinically significant morbidity but represent an opportunity for early intervention. When slowly progressive, cognitive functional loss can be difficult to detect. Moreover, formal assessment of cognition can be time consuming. Therefore, a staged approach is suggested with three levels; the first involves a very brief screen that takes only 2-3 minutes. This could take the form of a screening algorithm as developed by Cysique et al., or by paying close attention to “red flags,” as illustrated in (Fig. 1) If either of these is positive then the patient would be asked to return in another week or so for a longer screen. At this second level of assessment, the HIV dementia scale, CogState, or more detailed and focused questions could be used. This level of assessment would take approximately 10-15 minutes. If one of these was positive then the patient would be asked to return in another week or so for a formal evaluation of cognition (34). Although serial monitoring to detect cognitive changes may be useful for detection of dysfunction in early stages, it is important to consider a differential diagnosis that includes a variety of etiologies other than HAND. This includes depression and other psychological issues, metabolic disorders and opportunistic complications where relevant. It also includes the neurodegenerative diseases, such as Alzheimer’s and vascular cognitive impairment that may or may not be exacerbated by HIV infection.
Management
Antiretroviral agents with good penetration of the CSF produce lower CSF levels of viremia than agents with less penetration even when plasma viral load suppression is similar.
One of the most significant clinical issues is whether antiretroviral agents differ for their ability to prevent, reverse, or control HAND. To the degree that HAND is a direct consequence of neurotoxins generated by viral replication in the CNS, the major determinant of relative protection from antiretroviral therapies is likely to stem from their ability to penetrate the blood-brain barrier. Several studies have attempted to quantify this penetration using different techniques, such as quantifying drug levels in the CSF and evaluating on-treatment HIV suppression in the CSF (27). Antiretroviral agents with good penetration of the CSF produce lower CSF levels of viremia than agents with less penetration even when plasma viral load suppression is similar (35). A recently published study with more than 2600 patients associated antiretroviral regimens with good penetration with better neurocognitive function when this variable was assessed in a multivariate regression analysis (36). The optimal management of HAND is evolving. The primary focus should be viral suppression in both the blood and the CSF. Adjunctive therapies thus far have not been proven effective with the possible exception of memantine, which has had a modest degree of benefit (37-38). The presumed pathophysiology of cognitive loss makes control of HIV replication in the CSF an attractive theoretical target. There is support for this approach, even in those with undetectable viral load in the plasma, as approximately 10% of such patients will have detectable HIV replication in the CSF using standard assays (39). An even higher proportion will have detectable HIV RNA when single copy assays are used, but these are not in general use at present. In addition to a previously cited study in which antiretroviral therapy with good CSF penetration was associated with a reduction in CSF viral loads (27), another study associated a highly CSF-penetrating regimen with a reduction in cognitive deficit over a median 15-week follow-up relative to those receiving a less penetrative regimen (30). The substitution of antiretroviral agents with poor penetration, such as tenofovir, with agents that have good CSF penetration, such as abacavir, is an attractive strategy in patients experiencing cognitive loss. However, in those who are aviraemic in the plasma but detectable in the CSF, ARV resistance should be checked to guide therapy. In such cases, it may be better to add more highly-penetrating antiretrovirals than to substitute. The number of antiretrovirals to add is not known precisely but can be guided by the approach to HIV escape in the blood in HAART-treated patients where two new antiretrovirals are required. In HAND patients who are aviraemic in both the plasma and the CSF, optimal management is unknown. Intuitively, it would seem reasonable to add two highly-penetrating antiretrovirals to the existing regimen, but an evidence base for this is not available at present. The relative ability of this approach to reverse or preserve cognitive impairment may be dependent on the timing of the change in therapy, a specific reduction in HIV viral load in the CSF, or other variables. Additionally, non-drug related strategies may be helpful for slowing cognitive decline regardless of the pathophysiology. Mental and physical activity have demonstrated benefits in non-HIV patient groups, such as those with Alzheimer’s disease (40), and may be reasonable in the treatment of patients with HAND. Educating patients about the potential for cognitive decline and providing motivation to preserve cognitive function should not be overlooked as a potential intervention.
Conclusion
HAND has been a significant complication of HIV infection from the beginning of the epidemic. Although dementia is a risk factor for poor survival in patients with advanced HIV infection, cognitive impairment in patients whose plasma viremia is well controlled may represent a more subtle but still serious threat to long-term wellbeing. Due to the increasing proportion of HIV-infected individuals in many countries, including Canada, who are at risk for cognitive impairment due to long-term infection, advancing age, or both, the challenge of managing neurocognitive impairment in HIV care is expected to increase. While diseases associated with neurocognitive loss, such as Alzheimer’s, increase in incidence and prevalence among older individuals, HIV replication in the CSF appears to increase or accelerate neurocognitive loss. As a result, cognitive loss is encountered earlier and with greater frequency. Anticipation of these problems may be the first step toward improved management.
References
1. McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology 1993;43(11):2245-52. 2. Brew BJ, Dunbar N, Pemberton L, Kaldor J. Predictive markers of AIDS dementia complex: CD4 cell count and cerebrospinal fluid concentrations of beta 2-microglobulin and neopterin.J Infect Dis 1996;174(2):294-8. 3. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007;69(18):1789-99. 4. Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol 2002;8(2):136-42. 5. Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol 2004;10(6):350-7. 6. Heaton RK, Clifford DB, Franklin DR, Jr., et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75(23):2087-96. 7. Cysique LA, Maruff P, Brew BJ. Variable benefit in neuropsychological function in HIV-infected HAART-treated patients. Neurology 2006;66(9):1447-50. 8. Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology 2004;63(5):822-7. 9. Bhaskaran K, Mussini C, Antinori A, et al. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol 2008;63(2):213-21. 10. Foley J, Ettenhofer M, Wright MJ, et al. Neurocognitive functioning in HIV-1 infection: effects of cerebrovascular risk factors and age. Clin Neuropsychol 2010;24(2):265-85. 11. Rappaport J, Berger JR. Genetic testing and HIV dementia: teasing out the molecular mechanisms of disease. AIDS 2010;24(10):1585-7. 12. Hinkin CH, Castellon SA, Levine AJ, Barclay TR, Singer EJ. Neurocognition in individuals co-infected with HIV and hepatitis C. J Addict Dis 2008;27(2):11-7. 13. McGuire D. CSF biomarkers in HIV dementia: through a glass darkly. Neurology 2009;73(23):1942-4. 14. Sevigny JJ, Albert SM, McDermott MP, et al. An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. Arch Neurol 2007;64(1):97-102. 15. Gray F, Adle-Biassette H, Chretien F, Lorin de la Grandmaison G, Force G, Keohane C. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol 2001;20(4):146-55. 16. Ricardo-Dukelow M, Kadiu I, Rozek W, et al. HIV-1 infected monocyte-derived macrophages affect the human brain microvascular endothelial cell proteome: new insights into blood-brain barrier dysfunction for HIV-1-associated dementia. J Neuroimmunol 2007;185(1-2):37-46. 17. Strain MC, Letendre S, Pillai SK, et al. Genetic composition of human immunodeficiency virus type 1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy. J Virol 2005;79(3):1772-88. 18. Harezlak J, Buchthal S, Taylor M, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 2011. 19. Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J Neuroimmune Pharmacol 2010;5(3):294-309. 20. Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 2007;8(1):33-44. 21. Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 2005;19(4):407-11. 22. Herndon RM. Handbook of Neurologic Rating Scales. New York: Demos Medical Publishing; 2006. 23. Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011;17(1):3-16. 24. Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology 2008;22(1):110-7. 25. Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc 2002;8(3):410-24. 26. Brew BJ, Crowe SM, Landay A, Cysique LA, Guillemin G. Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol 2009;4(2):163-74. 27. Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008;65(1):65-70. 28. Letendre S, Ellis R, Deutsch R, et al. Correlates of time-to-loss-of-viral-response in CSF and plasma in the CHARTER cohort. In: Conference on Retroviruses and Opportunistic Infections (CROI); 2010; San Francisco; 2010. p. Abs 430. 29. Brew BJ, Halman M, Catalan J, et al. Factors in AIDS dementia complex trial design: results and lessons from the abacavir trial. PLoS Clin Trials 2007;2(3):e13. 30. Letendre SL, McCutchan JA, Childers ME, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol 2004;56(3):416-23. 31. Onen NF, Overton ET. A Review of Premature Frailty in HIV-infected Persons; Another Manifestation of HIV-Related Accelerated Aging. Curr Aging Sci 2011;4(1):33-41. 32. Orlando G, Meraviglia P, Cordier L, et al. Antiretroviral treatment and age-related comorbidities in a cohort of older HIV-infected patients. HIV Med 2006;7(8):549-57. 33. Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology 1991;41(6):778-85. 34. von Giesen HJ, Haslinger BA, Rohe S, Koller H, Arendt G. HIV Dementia Scale and psychomotor slowing–the best methods in screening for neuro-AIDS. J Neuropsychiatry Clin Neurosci 2005;17(2):185-91. 35. Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS 2009;23(11):1359-66. 36. Smurzynski M, Wu K, Letendre S, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS 2011;25(3):357-65. 37. Zhao Y, Navia BA, Marra CM, et al. Memantine for AIDS dementia complex: open-label report of ACTG 301. HIV Clin Trials 2010;11(1):59-67. 38. Brew BJ. Benefit or toxicity from neurologically targeted antiretroviral therapy? Clin Infect Dis 2010;50(6):930-2. 39. Eden A, Fuchs D, Hagberg L, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis 2010;202(12):1819-25. 40. Wilson RS, Barnes LL, Aggarwal NT, et al. Cognitive activity and the cognitive morbidity of Alzheimer disease. Neurology 2010;75(11):990-6.
Chapter 4: Neurocognitive Deficit
Cognitive decline is an insidious and frequently progressive complication of human immunodeficiency virus (HIV) infection. While the introduction of highly active antiretroviral therapy (HAART) largely eliminated the dementia associated with end-stage acquired immunodeficiency syndrome (AIDS), cognitive impairment remains a common complication of HIV even in those with well controlled viremia. The incidence and the severity of HIV-associated neurocognitive disorders (HAND) are rising with an aging HIV population – a potential consequence of both increased susceptibility in older individuals and progressive impairment with longer duration of infection. The clinical challenges posed by this complication are substantial. The relative ability of specific antiretroviral therapies to cross the blood brain barrier to reduce central nervous system (CNS) viral load, and the specific, perhaps genetic, susceptibility of individual patients are among factors that may explain the variable risk of cognitive impairment. In addition, HIV-independent variables may also be important in some individuals. Clinical sensitivity to changes in cognitive function through periodic assessments is appropriate in the context of strategies to understand and prevent HAND.
Show review