Expert Review
The Aging HIV Patient
Chapter 1: Aging in HIV – Overview
Anita Rachlis, MD, MEd, FRCPC
University of Toronto, Toronto, ON
Age-related diseases, such as atherosclerosis and osteoporosis, are being observed at a younger age in patients infected with human immunodeficiency virus (HIV) than in those without infection. The apparent acceleration in aging may be related to several causes, including persistent upregulation of the inflammatory response and the adverse effects of antiretroviral therapies. The acceleration of aging processes threaten to shorten the lifespan of patients with HIV even when immune function has been improved and the viremia remains optimally suppressed. The individual risk for specific diseases varies, but it has become important to direct attention toward opportunities to modify or circumvent the potential for irreversible damage to target organs. In general, the average age for symptomatic manifestations of processes common to aging individuals, such as bone mineral loss and neurocognitive decline, appears to be at least one decade earlier when those with HIV infection are compared to those without. In Canada, where the population of HIV-positive individuals over the age of 50 is increasing, strategies for anticipating and modifying these risks are expected to be an increasingly important part of HIV management.
Aging of the HIV Population: Epidemiology
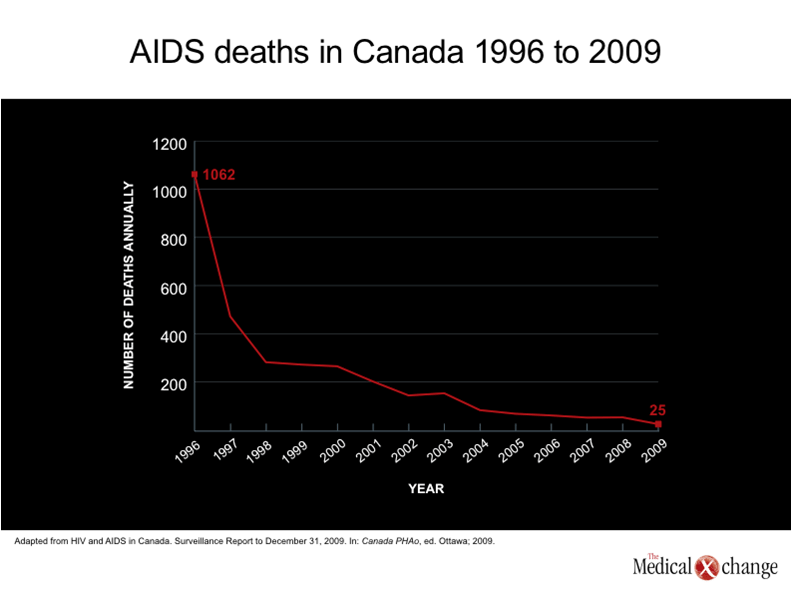
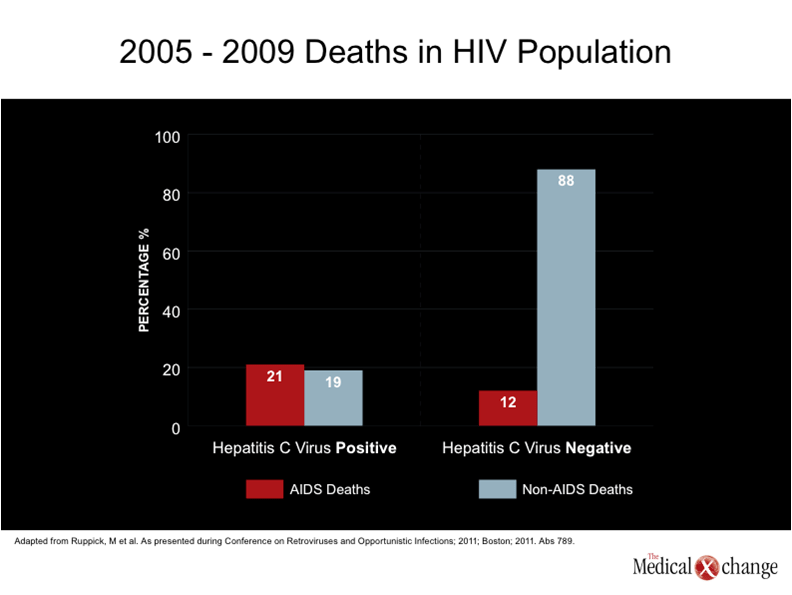
In Canada, like many other countries where antiretroviral agents are readily available, the ability of current therapies to sustain control of human immunodeficiency virus (HIV) has led to a growing number of patients who are 50 years old or older.(1-2)This age stratification, used by the Public Health Agency of Canada for tracking the epidemiology of HIV and acquired immunodeficiency syndrome (AIDS),(3)is a potentially useful threshold because of increasing evidence that HIV may pose unique challenges in aging patients. Older patients have long been over-represented among AIDS cases relative to their total representation among patients with HIV,(1)but there is growing concern that HIV infection may complicate or even accelerate the co-morbidities common to the aging process,(4)such as cardiovascular disease (CVD),(5)renal impairment,(6)osteoporosis,(7)liver disease,(8)and neurocognitive decline.(9) The aging of the HIV population in Canada can be understood in two different ways. One is the increasing proportion of new infections in patients who are 50 years old or older. Individuals over the age of 50 now represent close to 15% of new infections in Canada, a proportion that has almost doubled since the late 1990s.(3)The other is the prolonged survival made possible with highly active antiretroviral therapies (HAART). While the proportion of HIV-positive patients who are older than age 50 years increased very modestly in Canada over the last 10 years because the majority of new infections still occur in younger individuals,(10)the absolute number of older individuals with HIV is increasing along with the increasing estimated number of total infections, which exceeded 70,000 persons by the end of 2010.(1) While persistent and sustained suppression of HIV remains critical to the survival of patients with HIV, AIDS deaths are now infrequent in Canada. In 2009, there was a total of 25 deaths attributed to AIDS, which represented an 84% reduction since 2003 despite a substantial increase in the number of individuals living with HIV.(10) (Fig. 1). Similar reductions have been recently reported in other countries with ready access to antiretroviral therapies, such as Switzerland.(11)In the Swiss HIV Cohort Study (SHCS), less than 20% of all deaths in 2005 through 2009 were due to AIDS. Rather, non-AIDS malignancies followed by CVD were the most common causes of death in patients without hepatitis C virus (HCV) infection. Liver disease, followed by non-AIDS infections were the most common causes of mortality in HIV patients co-infected with HCV. Over this study period, the median age at death climbed from 45 to 49 years while the median CD4 count at time of death increased from 257 to 321 cells/mm3 (Fig. 2). These data suggest that prolonging survival in patients with HIV is becoming less dependent on developing better therapies for suppressing HIV than controlling disease processes that are exacerbated by the presence of HIV or its treatments. The risks for specific diseases, such as those affecting the CV system, the central nervous system (CNS), or the skeleton, may range substantially among HIV-infected individuals due to an array of variables that affect specific risk, including familial susceptibility, lifestyle choices, and medication history, but current data suggest that the treatment of these co-morbid conditions will be increasingly critical to the effort to extend the life span of individuals infected with HIV.
Pathogenesis
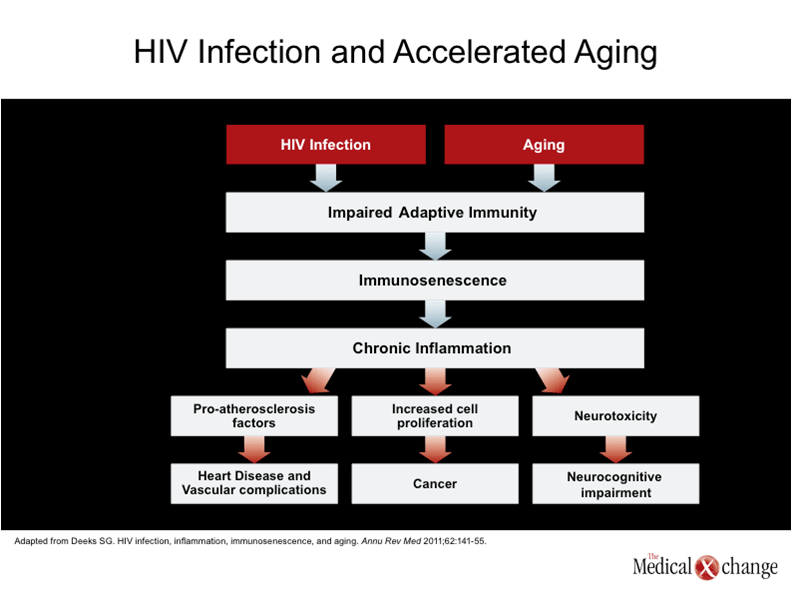
The association between HIV infection and an increased risk of CVD, which is well described and one of the first signals that age-related diseases occur earlier in patients with HIV,(12-14)was not initially recognized as a manifestation of accelerated aging. Rather, the initial focus was on the dyslipidemias associated with protease inhibitors (PIs) and the high prevalence of conventional heart disease risk factors, such as smoking, in the HIV-infected population.(15-16)Those factors remain relevant, but numerous other factors appear to be contributing, including a direct influence of HIV on CV risk, and that this risk may in part be mediated by immunosenescence.(17)The concept of immunosenescence, which is relevant to natural aging, is based on a decline in the adaptive immune system that, among other effects, leads to upregulation of inflammatory mediators.(18)In CVD, it is now believed that inflammation participates in promoting both atherosclerosis and thrombotic events.(19-20) A similar interplay between an upregulation of the immune system and accelerated senescence may be relevant to other organ systems in patients with HIV.(21)The aging process, which is generally recognized as a progressive cumulative deterioration in normal physiologic molecular and cellular functions resulting in organ damage, has been linked to expression of several specific inflammatory cytokines, such as interleukin-6 (IL-6), in normal as well as HIV-associated aging.(22-23)This has led to the hypothesis that HIV patients are aging more quickly simply because the persistent infection exhausts the immune system at a more rapid pace.(17) (Fig. 3). In normal aging, the rate of impairment in specific organs depends on multiple factors, particularly genetics. Genetic variables that affect risk have been defined for CVD,(24)neurocognitive impairment,(25)osteoporosis,(26)and renal impairment.(27)Although lifestyle choices may have an important influence on the relative risk of all of these processes, suggesting that genetic predisposition can be exacerbated or reduced, the risk of death from any single cause of death appears to vary widely. Current efforts to determine the relative contribution of DNA damage and repair, telomere shortening, and mitochondrial dysfunction may all provide insight on individual differences in the rate of aging in specific organs.(28)All of these factors are likely to be relevant to the relative rate of deterioration in specific organ systems in those who are HIV infected.
Clinical Features
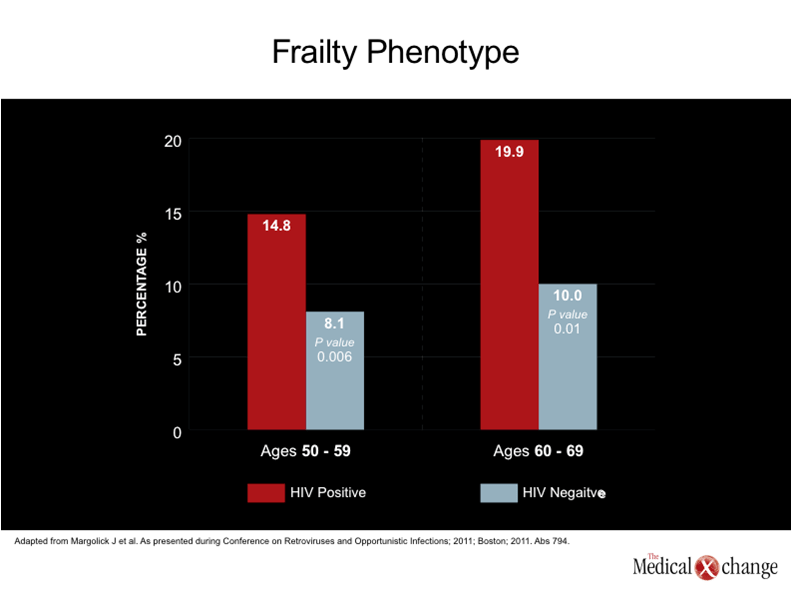
The cumulative effect of the aging process in normal individuals is a generalized frailty, which is also observed in individuals with HIV. As anticipated by an accelerated rate of aging, the increasingly well-defined frailty phenotype occurs at a much younger age in individuals who are HIV positive. (Fig. 4) In new data from the Multicenter AIDS Cohort Study (MACS), pre-defined frailty characteristics were almost twice as common in men with HIV relative to men without HIV between the ages of 50 and 59 (14.8 vs. 8.1%; P=0.006) and 60 and 69 (19.9 vs. 10.0%; P=0.01).(29)In this study, individuals were considered to have frailty if they had three or more of the following: low grip strength (20th percentile for age), slow four-meter walk (<20th percentile for age), low physical activity, exhaustion as defined by the Short-Form 36 (SF-36), and unintentional weight loss of >10 pounds. Traditionally, the goals of HIV therapy have been to suppress viremia while minimizing the potential for adverse effects from the medications. The evidence of accelerated aging, suggests a new paradigm that includes attention to target organs at risk.(30)While a specific definition of frailty in the HIV-positive patient may be helpful to better understand this phenomenon, criteria for early recognition of the process is supported by the association of frailty with adverse outcomes in individuals who are HIV-negative.(31)
Diagnosis and Monitoring
The evidence that HIV accelerates aging promotes a proactive approach to patient care. Unlike acute health complaints, age-related deterioration in organ systems can be subtle and insidious. Many of the processes, including CVD and renal impairment, may not elicit direct complaints but remain asymptomatic up until life-threatening complications occur. It is therefore appropriate to consider an organized approach to gathering baseline information about CV risk factors, renal function, cognitive function, and bone mineral density. It is essential to recognize that aging processes in all of these organ systems are multifactorial. For example, while dyslipidemias were once considered to be most likely explanation for the relationship between antiretroviral therapy and CVD,(32)exposure to protease inhibitors, nucleoside reverse transcriptase inhibitors (NRTIs) specifically and antiretroviral agents overall have also been associated with increased insulin resistance, an independent risk factor for CVD.(33-34) HIV-infected individuals face the same health risks as non-infected individuals, but the interrelationship of normal health risks, accelerated aging, and the effects of antiretroviral therapy defines a need to initiate more rigorous monitoring of age-related risks at a much earlier timepoint than would be appropriate in individuals without HIV infection. Due to the multiple pathophysiologic processes at work in HIV-infected individuals, each risk factor may threaten a greater impact than anticipated in an individual without HIV. For example, therapeutic agents designed to reverse the complex effect of HIV infection on visceral fat, insulin resistance, and lipodystrophy, are being pursued for their potential to circumvent a synergy between these risk factors.(17)
Management
Evidence of risk in specific organ systems can be addressed with targeted therapies according to evidence-based guidelines, such as those provided by the Canadian Cardiovascular Society for CV risk factors or the Canadian Society of Nephrology for the management of chronic kidney disease.(35-36)However, the best opportunity for reducing the morbidity and mortality in aging patients with HIV may be to intervene in advance of pathology. Although strategies must be individualized for the individual risk profiles, aggressive interventions at a relatively young age, including lifestyle changes, may have a large impact on subsequent risk. In this setting, family history, in addition to baseline monitoring, can be helpful for anticipating risks. Although no objective takes precedence over sustained suppression of HIV, the relative safety of antiretroviral agents for lipid metabolism or kidney function may be relevant in patients with elevated risk for CVD or kidney disease, respectively. Recent attention to the relative penetration of antiretroviral agents into the CNS compartment may prove relevant to relative risk reduction for neurocognitive impairment,(37)although more studies are required to understand the potential for neurotoxicity among antiretroviral agents and to confirm the relative clinical value of agents that do or do not have a high central nervous system penetration effectiveness (CPE) score. Ultimately, strategies for optimal management of age-related diseases are likely to evolve as the growing number of older HIV-positive patients expands the scope of this clinical challenge.
Conclusion
With the identification of numerous effective antiretroviral combinations that provide sustained control of HIV, age-related diseases will increasingly represent the primary obstacle to a normal lifespan among infected individuals. The relative risks of specific organ diseases appear to be patient-specific, but the vulnerability may have a common pathway related to immunosenescence and the persistent upregulation of the inflammatory response induced by the presence of HIV. Strategies for an individualized but proactive approach to slowing decline in susceptible organs are emerging, but this is a dynamic area which is expected to evolve.
References
1. HIV/AIDS Epi Updates – July 2010. Public Health Canada, 2010. (Accessed March 7, 2011, 2011, at http://www.phac-aspc.gc.ca/aids-sida/publication/epi/2010/1-eng.php.) 2. Mack KA, Ory MG. AIDS and older Americans at the end of the Twentieth Century. J Acquir Immune Defic Syndr 2003;33 Suppl 2:S68-75. 3. PublicHealthCanada. HIV/AIDS Epi Updates, November 2007. In: Canada PHAo, ed. 2007. Ottawa; 2007. 4. Kearney F, Moore AR, Donegan CF, Lambert J. The ageing of HIV: implications for geriatric medicine. Age Ageing 2010;39(5):536-41. 5. Sudano I, Spieker LE, Noll G, Corti R, Weber R, Luscher TF. Cardiovascular disease in HIV infection. Am Heart J 2006;151(6):1147-55. 6. Winston J, Deray G, Hawkins T, Szczech L, Wyatt C, Young B. Kidney disease in patients with HIV infection and AIDS. Clin Infect Dis 2008;47(11):1449-57. 7. Thomas J, Doherty SM. HIV infection–a risk factor for osteoporosis. J Acquir Immune Defic Syndr 2003;33(3):281-91. 8. Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166(15):1632-41. 9. McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol 2004;157(1-2):3-10. 10. HIV and AIDS in Canada. Surveillance Report to December 31, 2009. In: Canada PHAo, ed. Ottawa; 2009. 11. Ruppick M, Ledergerber B, Rickenbach M, Furrer H, Battegay M. Changing patterns of causes of death: SHCS, 2005 to 2009. In: Conference on Retroviruses and Opportunistic Infections; 2011; Boston; 2011. 12. Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med 2006;145(6):397-406. 13. Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007;356(17):1723-35. 14. Obel N, Thomsen HF, Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis 2007;44(12):1625-31. 15. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92(7):2506-12. 16. Stein JH, Hadigan CM, Brown TT, et al. Prevention strategies for cardiovascular disease in HIV-infected patients. Circulation 2008;118(2):e54-60. 17. Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011;62:141-55. 18. Molina-Pinelo S, Vallejo A, Diaz L, et al. Premature immunosenescence in HIV-infected patients on highly active antiretroviral therapy with low-level CD4 T cell repopulation. J Antimicrob Chemother 2009;64(3):579-88. 19. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352(16):1685-95. 20. Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol 2007;14(1):55-61. 21. Onen NF, Overton ET. A review of premature frailty in HIV-infected persons; another manifestation of HIV-related accelerated aging. Curr Aging Sci 2011;4(1):33-41. 22. Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 2002;162(20):2333-41. 23. Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008;5(10):e203. 24. Humphries SE, Ridker PM, Talmud PJ. Genetic testing for cardiovascular disease susceptibility: a useful clinical management tool or possible misinformation? Arterioscler Thromb Vasc Biol 2004;24(4):628-36. 25. Lambert JC, Amouyel P. Deciphering genetic susceptibility to frontotemporal lobar dementia. Nat Genet 2010;42(3):189-90. 26. Mitchell BD, Yerges-Armstrong LM. The Genetics of Bone Loss: Challenges and Prospects. J Clin Endocrinol Metab 2011. 27. Bowden DW. Genetics of kidney disease. Kidney Int Suppl 2003(83):S8-12. 28. Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 2010;464(7288):520-8. 29. Margolick J, Li X, Detels R, Phair J, Rinaldo C, Jacobson L. Earlier occurrence of the frailty phenotype in HIV+ men than in HIV- men: the MACS. In: Conference on Retroviruses and Opportunistic Infections; 2011; Boston, Massachusetts; 2011. 30. Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep 2010;7(2):69-76. 31. Repetto L, Venturino A, Fratino L, et al. Geriatric oncology: a clinical approach to the older patient with cancer. Eur J Cancer 2003;39(7):870-80. 32. Rhew DC, Bernal M, Aguilar D, Iloeje U, Goetz MB. Association between protease inhibitor use and increased cardiovascular risk in patients infected with human immunodeficiency virus: a systematic review. Clin Infect Dis 2003;37(7):959-72. 33. Tien PC, Schneider MF, Cole SR, et al. Antiretroviral therapy exposure and insulin resistance in the Women’s Interagency HIV study. J Acquir Immune Defic Syndr 2008;49(4):369-76. 34. Stein JH. Dyslipidemia in the era of HIV protease inhibitors. Prog Cardiovasc Dis 2003;45(4):293-304. 35. Genest J, McPherson R, Frohlich J, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult – 2009 recommendations. Can J Cardiol 2009;25(10):567-79. 36. Levin A, Hemmelgarn B, Culleton B, et al. Guidelines for the management of chronic kidney disease. CMAJ 2008;179(11):1154-62. 37. Arendt G, Orhan E, Nolting T. Retrospective analysis of the HAART CPE index on neuropsychological performance of a big NeuroAIDS cohort. In: Conference on Retroviruses and Opportunistic Infections; 2011; Boston, Massachusetts; 2011.
Chapter 1: Aging in HIV – Overview
Age-related diseases, such as atherosclerosis and osteoporosis, are being observed at a younger age in patients infected with human immunodeficiency virus (HIV) than in those without infection. The apparent acceleration in aging may be related to several causes, including persistent upregulation of the inflammatory response and the adverse effects of antiretroviral therapies. The acceleration of aging processes threaten to shorten the lifespan of patients with HIV even when immune function has been improved and the viremia remains optimally suppressed. The individual risk for specific diseases varies, but it has become important to direct attention toward opportunities to modify or circumvent the potential for irreversible damage to target organs. In general, the average age for symptomatic manifestations of processes common to aging individuals, such as bone mineral loss and neurocognitive decline, appears to be at least one decade earlier when those with HIV infection are compared to those without. In Canada, where the population of HIV-positive individuals over the age of 50 is increasing, strategies for anticipating and modifying these risks are expected to be an increasingly important part of HIV management.
Show review