Expert Review
The Aging HIV Patient
Chapter 5: Osteoporosis
Greg Bondy, MSc, MD, FRCPC
University of British Columbia, Vancouver, BC
The accelerated loss of bone density in patients with HIV infection threatens a significant health crisis in Canada and other countries with aging HIV-infected populations. There appears to be an important interaction between traditional osteoporosis risk factors and bone loss related specifically to HIV and its therapies. Consistent with accelerated aging across other organ systems, HIV-related loss of bone mineral density is a progressive condition detected soon after infection. It may persist independent of HIV suppression, and it can be exacerbated by some antiretroviral drugs. Strategies to diminish the impact of bone mineral loss depend on early screening and aggressive efforts at preventing or modifying the underlying processes. The rising rates of fracture in aging individuals with HIV infection have intensified attention on this complication, but the scope of this complication is expected to enlarge with the demographic shift that is increasing the proportion of HIV-infected individuals in the age range of vulnerability.
Osteoporosis in HIV: Epidemiology
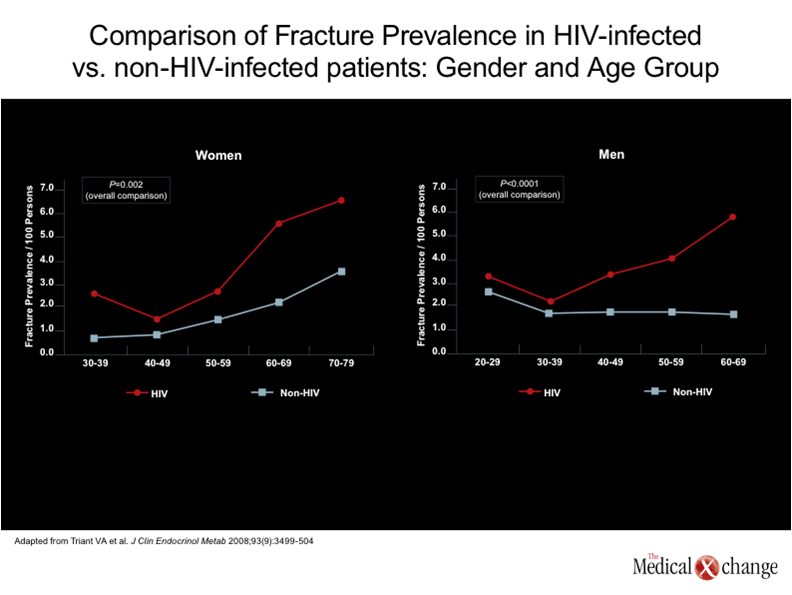
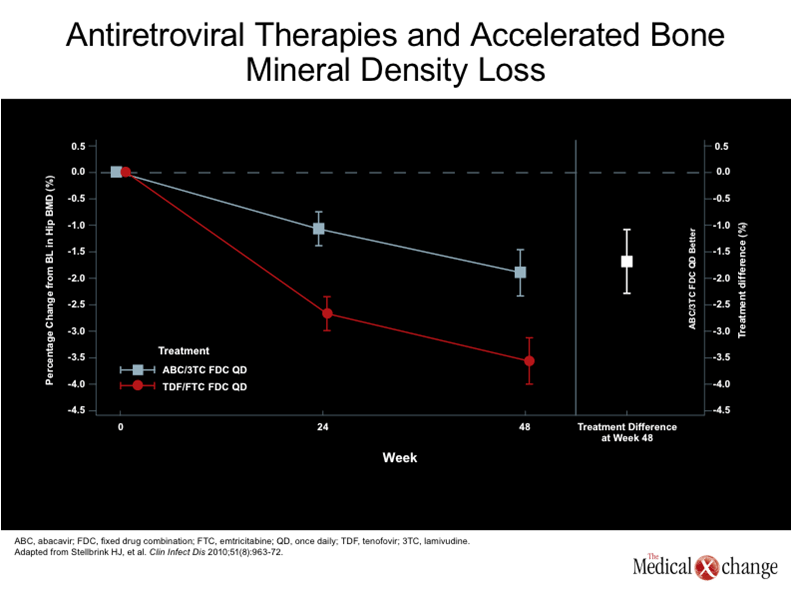
Osteoporosis is a significant public health problem independent of HIV infection. Although the prevalence is higher in postmenopausal women, the rates of bone loss are similar in women and men after about age 60.(1)In Canada, where the population is aging,(2)it is estimated that one in four women and one in eight men have osteopenia that poses and increased risk of bone fracture.(3-4)Data from the United States suggest that 50% of individuals have developed osteoporosis by the time they reach the age of 80 years.(5)In patients with HIV, the incidence of osteoporosis appears to begin at a much earlier age and the prevalence climbs more steeply.(6)(see Figure 1) Although it was initially hypothesized that osteoporosis might primarily be a complication of antiretroviral therapy,(7)HIV infection is now recognized as an independent risk factor for bone disease.(8)While specific antiretroviral therapies do appear to accelerate the process, osteopenia begins to develop early in the infection and in the absence of therapy.(6, 9)Within two years of HIV infection, bone density declines by as much as 6%,(10)which is similar to that observed among women within the first two years of menopause.(11) The definition of osteopenia in individuals greater than 50 years of age is more than 1.0 but less than 2.5 standard deviations below the average bone mineral density (BMD) of adults between the ages of 25 and 35, according to the World Health Organization (WHO).(12)This is typically referred to as a T score. The definition of osteoporosis is 2.5 standard deviations below average. Both osteopenia and osteoporosis can also be defined by Z scores, which evaluate standard deviations related to sex- and ethnicity-matched populations of the same age. More commonly used in younger individuals, including those infected with HIV, an abnormal Z score is considered to be 2.0 standard deviations below the benchmark.(13)There is a steep increase in fracture rate with each standard deviation below the mean.(14) The risk for fracture among individuals with HIV varies based on numerous risk factors, but population-based studies suggest that the overall increase in risk is large. In one study that compared fracture rates among 8525 HIV-infected and 2,208,792 non-infected individuals, the fracture prevalence calculated per 100 years was 2.87 for those with HIV vs. 1.77 among the comparator group.(15)This 62.1% increase in rate was highly statistically significant (P<0.0001). Although the increased risk of specific fractures was not evenly distributed by site (for example, vertebral facture rates were nearly twice as great in women with HIV, but hip fractures were not significantly different), the overall increase in fracture prevalence among HIV-infected males was even greater (68.3%; P<0.0001) than in females (44.7%; P=0.002) (Fig. 1). Specific antiretroviral agents have been shown to exacerbate of the bone mineral density loss associated with HIV. In a substudy of the ANRS 121 study, which randomized HIV-infected individuals to a regimen that included a ritonavir-boosted protease inhibitor (PI/r), a non-nucleoside reverse transcriptase inhibitor (NNRTI) or both, the mean reduction in bone density in the lumbar spine was significantly more on the PI/r arm (-5.8%; P=0.007) and the PI/r and NNRTI combination arm (-4.4%; P=0.001) than on the NNRTI only arm (-1.5%).(16) The strongest association between antiretroviral therapy and risk of accelerated bone mineral density loss has been with tenofovir (TDF). In a multicenter 385-patient, 96-week study comparing TDF in combination with emtricitabine (FTC) to abacavir (ABC) in combination with lamivudine (3TC), the mean change from baseline was 1.9% in the group receiving ABC/3TC and -3.6% in those receiving TDF/FTC.(17) (Fig. 2). This 89.4% increase was highly statistically significant (P<0.001). Moreover, when bone loss greater than 6% was compared at specific sites, many showed loss that was three or four times greater in patients receiving the TDF-containing regimen rather than the combination with ABC. This included the hip (13% vs. 3%) and the spine (15% vs. 5%). Previous controlled studies have also associated TDF exposure with increased risk of bone density loss.(18-19) The aging of the HIV population predicts rising rates of fractures, but the consequences of bone mineral density loss are progressive, suggesting that early prevention may modify clinical risks. While the rising rates of fracture in individuals with long-term HIV infection demonstrate that bone mineral density loss is an important challenge, the complications of osteoporosis have the potential to reduce survival in aging HIV-infected individuals.
Pathogenesis
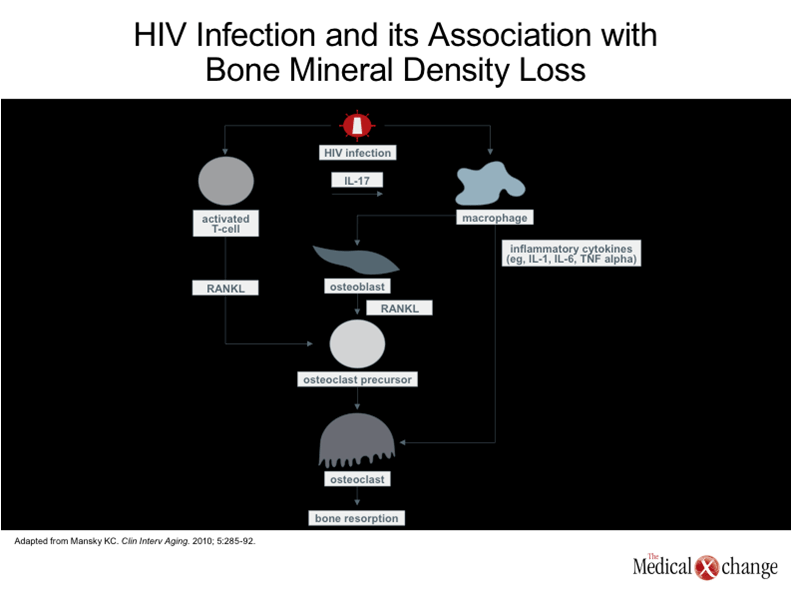
Bone metabolism is a dynamic process of bone remodeling in which stable bone density is dependent on a balance between the bone resorption provided by osteoclasts and bone formation by osteoblasts.(20)Although low accumulation of peak bone mass, which is reached in late adolescence or early adulthood,(21)can be a susceptibility factor for osteopenia later in life, the pace of bone mineral density loss over the course of adulthood is influenced by a broad array of factors, including nutrition, exercise, hormone levels, and factors that influence bone metabolism signaling.(22) While HIV-infected patients are susceptible to the same risks for osteoporosis as those without HIV, including poor nutrition and genetic susceptibility, HIV and its therapies have unique effects on bone metabolism. Perhaps most importantly, HIV proteins have been shown to promote osteoblast apoptosis, inhibiting bone formation,(23)while both HIV proteins and inflammatory cytokines associated with HIV, infection, such as tumor necrosis factor alpha (TNF-α), have been associated with increased osteoclast activity and bone resorption.(23) (Fig. 3). These activities are consistent with the evidence that significant bone loss begins soon after infection irrespective of the type of therapy or whether therapy is initiated.(10) In experimental studies, antiretroviral therapies have demonstrated a variety of potential adverse effects on bone metabolism. For example, efavirenz, an NNRTI, has been associated with an impairment of hepatic enzymes important to vitamin D metabolism, while some PIs, such as nelfinavir and ritonavir, increase gene expression of selected pro-inflammatory cytokines, such as interleukin-8 (IL-8), that adversely affect osteoblasts. However, the association of these agents and exacerbation of bone loss beyond that produced by HIV has been inconsistent.(24-25) The association of TDF and bone loss, demonstrated in a prospective, randomized controlled trial,(17)has been far more consistent. Several mechanisms may be involved, including alterations in gene expression that control osteoblast and osteoclast activity.(26)TDF, a phosphonate with the potential to be taken up by osteoclasts, may also induce stress that alters reciprocal signaling important to osteoblast activity.(27)In addition, TDF is associated with impairment of renal function,(28)which is a risk factor for osteoporosis.(29)The interaction of TDF with conventional risk factors for osteoporosis deserves further study. In addition, osteoporosis in patients with HIV may be understood as a consequence of an accelerated aging phenomenon related to immunosenescence.(30)The premise of immunosenescence is that progressive functional impairment in the immunoregulatory system associated with age is the basis for a broad array of diseases that become more common in aging adults, including cancer, atherosclerosis, and osteoporosis.(31)Due to the stress placed on the immune system by HIV infection, this age-related process appears to begin earlier and progress more rapidly.(32)
Diagnosis and Monitoring
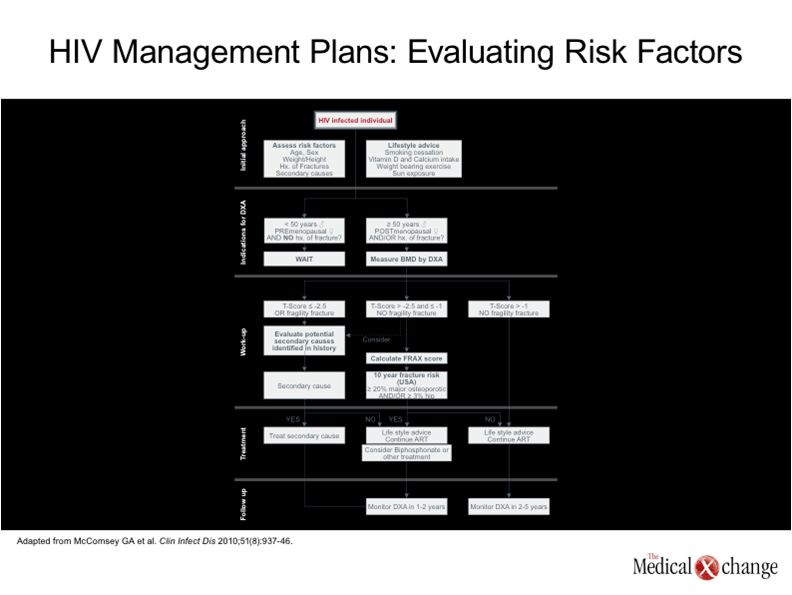
Due to the broadly shared risk of osteoporosis in aging individuals, screening for bone mineral density is recommended in all Canadians 65 years of age or older.(33)The age for screening is lower to 50 years for both men and menopausal women with risk factors. These risk factors include a fragility fracture after the age of 40, a family history of osteoporosis, current smoking, high alcohol intake, a low body weight, or disorders associated with osteoporosis, such as rheumatoid arthritis, type 1 diabetes, or chronic inflammation. Screening is recommended in younger adults who have had a fragility fracture, prolonged use of glucocorticoids, or diseases associated with osteoporosis, such as hyperparathyroidism. Screening is performed with bone mineral density evaluations typically conducted with dual x-ray absorptiometry (DEXA). The Osteoporosis Canada guidelines do not specifically identify HIV as a risk factor, but other organizations, such as the Infectious Diseases Society of American (IDSA) have, advocating DEXA scans in all HIV patients with risk factors beginning at 50 years.(34)However, in a more recently published multinational collaborative review, scanning at the age of 50 years was recommended in all patients with HIV regardless of risk factors.(8)This group, comprised largely of clinicians and researchers active in the field of HIV, recommended repeating the test every two to five years. Although screening earlier than age 50 is not routinely recommended, any history of fragility fractures, whether or not patients have HIV, is an indication for a DEXA scan.
Management
In patients with clinically significant bone density loss as defined by T or Z scores, a complete evaluation to determine risk factors is essential to develop an appropriate management plan. (Fig. 4). While alcoholism, glucocorticoid exposure, and hypogonadism are common risk factors for osteoporosis in general,(35)risk factors for osteoporosis that have been specifically identified to be common in patients with HIV include low body weight, insulin resistance, and hyperlactatemia (36-37). A thorough examination to identify secondary causes of osteoporosis should include routine blood chemistry tests, renal function tests, serum hydroxyvitamin D level determinations, and appropriate hormone level tests by gender. While secondary causes of osteoporosis should be addressed directly, acute or chronic nutritional and pharmacologic therapies are appropriate to improve bone metabolism. It is reasonable to employ the same treatments in patients with HIV as in those who do not have HIV, although the data demonstrating benefit in the setting of HIV is generally more limited. In addition to ensuring adequate levels of calcium and vitamin D intake, sun exposure, and exercise, this can includes bisphosphonates, which have been shown to reduce the risk of fracture in individuals without HIV.(38)In patients with HIV, bone density has improved in patients in a placebo-controlled trial of the bisphosphonate zoledronate, although follow-up was not sufficient to demonstrate an effect on fracture risk.(39) Selecting an antiretroviral therapy with minimal influence on bone metabolism may also be appropriate. Although sustained suppression of HIV is the single most important priority for preventing life-threatening complications of HIV, there is now strong evidence that TDF increases the risk of bone mineral density loss. Although the evidence that TDF increases the risk of fracture is weaker, it may be appropriate to institute closer monitoring of HIV-infected patients with risk factors for osteoporosis who are taking a regimen that contains TDF or to consider alternatives in those who already have osteoporosis. Prevention of osteoporosis in patients with HIV has not been well explored. However, due to the high risk of this complication, it is reasonable to consider prophylactic strategies, such as adequate exercise and intake of nutrients important to bone metabolism, even in young adults. Such lifestyle modifications should be implemented immediately in those individuals with osteopenia even if pharmacologic therapies are not yet indicated. In patients with osteoporosis, DEXA scans should be considered one to two years after initiating a treatment program so that pharmacologic therapies, which are not without adverse effects or costs,(40)can be stopped if adequate bone density has been restored.
Conclusion
Osteoporosis is a major health threat in aging individuals with HIV infection. Bone mineral density loss and clinical complications of osteoporosis can be observed a decade or more earlier in individuals with HIV than in the general population. The HIV infection appears to impose direct adverse effects on bone metabolism, but the increased rate of osteoporosis is multifactorial and may be part of a syndrome of frailty in individuals with HIV driven by accelerated immunosenescence. Osteoporosis in patients with HIV does appear to be modifiable by addressing risk factors, employing pharmacologic therapies that increase bone density, and reducing exposure to antiretroviral agents that exacerbate bone loss. Greater attention to this problem may be essential to efforts to extend survival in HIV-infected patients who are otherwise well controlled on their antiretroviral therapies.
Conclusion
1. O’Flaherty EJ. Modeling normal aging bone loss, with consideration of bone loss in osteoporosis. Toxicol Sci 2000;55(1):171-88. 2. Canada’s Aging Population. Health Canada, 2009. (Accessed February 16, 2011, at http://dsp-psd.pwgsc.gc.ca/Collection/H39-608-2002E.pdf.) 3. Hanley DA, Josse RG. Prevention and management of osteoporosis: consensus statements from the Scientific Advisory Board of the Osteoporosis Society of Canada. 1. Introduction. CMAJ 1996;155(7):921-3. 4. Jackson SA, Tenenhouse A, Robertson L. Vertebral fracture definition from population-based data: preliminary results from the Canadian Multicenter Osteoporosis Study (CaMos). Osteoporos Int 2000;11(8):680-7. 5. Looker AC, Orwoll ES, Johnston CC, Jr., et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res 1997;12(11):1761-8. 6. Bruera D, Luna N, David DO, Bergoglio LM, Zamudio J. Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS 2003;17(13):1917-23. 7. Knobel H, Guelar A, Vallecillo G, Nogues X, Diez A. Osteopenia in HIV-infected patients: is it the disease or is it the treatment? AIDS 2001;15(6):807-8. 8. McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis 2010;51(8):937-46. 9. Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 2006;20(17):2165-74. 10. Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr 2009;51(5):554-61. 11. Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 2008;93(3):861-8. 12. WHO. Assessment of fracture risk and its application to screening for post-menopausal osteoporosis. World Health Organ Tech Rep Ser 1994;843:1-129. 13. NOF. Clinician’s Guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation 2010. 14. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996;312(7041):1254-9. 15. Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab 2008;93(9):3499-504. 16. Duvivier C, Kolta S, Assoumou L, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS 2009;23(7):817-24. 17. Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 2010;51(8):963-72. 18. Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004;292(2):191-201. 19. Grund B, Peng G, Gibert CL, et al. Continuous antiretroviral therapy decreases bone mineral density. AIDS 2009;23(12):1519-29. 20. Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med 2005;11(2):76-81. 21. Mora S, Gilsanz V. Establishment of peak bone mass. Endocrinol Metab Clin North Am 2003;32(1):39-63. 22. Rahmani P, Morin S. Prevention of osteoporosis-related fractures among postmenopausal women and older men. CMAJ 2009;181(11):815-20. 23. Gibellini D, De Crignis E, Ponti C, et al. HIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNFalpha activation. J Med Virol 2008;80(9):1507-14. 24. Fabbriciani G, De Socio GV. Efavirenz and bone health. AIDS 2009;23(9):1181. 25. Amiel C, Ostertag A, Slama L, et al. BMD is reduced in HIV-infected men irrespective of treatment. J Bone Miner Res 2004;19(3):402-9. 26. Grigsby IF, Pham L, Mansky LM, Gopalakrishnan R, Carlson AE, Mansky KC. Tenofovir treatment of primary osteoblasts alters gene expression profiles: implications for bone mineral density loss. Biochem Biophys Res Commun 2010;394(1):48-53. 27. Grigsby IF, Pham L, Mansky LM, Gopalakrishnan R, Mansky KC. Tenofovir-associated bone density loss. Ther Clin Risk Manag 2010;6:41-7. 28. Zimmermann AE, Pizzoferrato T, Bedford J, Morris A, Hoffman R, Braden G. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin Infect Dis 2006;42(2):283-90. 29. Jassal SK, von Muhlen D, Barrett-Connor E. Measures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: the Rancho Bernardo study. J Bone Miner Res 2007;22(2):203-10. 30. Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011;62:141-55. 31. Pawelec G. Immunosenescence. Philadelphia: Springer; 2007. 32. Molina-Pinelo S, Vallejo A, Diaz L, et al. Premature immunosenescence in HIV-infected patients on highly active antiretroviral therapy with low-level CD4 T cell repopulation. J Antimicrob Chemother 2009;64(3):579-88. 33. Papaioannou A, Morin S, Cheung AM, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 2010;182(17):1864-73. 34. Aberg JA, Kaplan JE, Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2009;49(5):651-81. 35. Cohen A, Shane E. Primer on the metabolic bone diseases and other disorders of bone and mineral metabolism: premenopausal osteoporosis: J.W. Wiley; 2008. 36. Chew NS, Doran PP, Powderly WG. Osteopenia and osteoporosis in HIV: pathogenesis and treatment. Curr Opin HIV AIDS 2007;2(4):318-23. 37. Pollock E, Klotsas AE, Compston J, Gkrania-Klotsas E. Bone health in HIV infection. Br Med Bull 2009;92:123-33. 38. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med 2009;122(2 Suppl):S14-21. 39. Huang J, Meixner L, Fernandez S, McCutchan JA. A double-blinded, randomized controlled trial of zoledronate therapy for HIV-associated osteopenia and osteoporosis. AIDS 2009;23(1):51-7. 40. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 2005;90(3):1294-301.
Chapter 5: Osteoporosis
The accelerated loss of bone density in patients with HIV infection threatens a significant health crisis in Canada and other countries with aging HIV-infected populations. There appears to be an important interaction between traditional osteoporosis risk factors and bone loss related specifically to HIV and its therapies. Consistent with accelerated aging across other organ systems, HIV-related loss of bone mineral density is a progressive condition detected soon after infection. It may persist independent of HIV suppression, and it can be exacerbated by some antiretroviral drugs. Strategies to diminish the impact of bone mineral loss depend on early screening and aggressive efforts at preventing or modifying the underlying processes. The rising rates of fracture in aging individuals with HIV infection have intensified attention on this complication, but the scope of this complication is expected to enlarge with the demographic shift that is increasing the proportion of HIV-infected individuals in the age range of vulnerability.
Show review