Nephrology
Hyperphosphatemia: Expert Review and Commentary from Published Literature
Hyperphosphatemia in Dialysis: Strategies for Maintaining Target Serum Phosphate Levels
Robert Ting, MD, FRCP(C), FACP
Nephrologist
Scarborough Health Network (SHN)
Medical Director, Dialysis Management Clinics
Toronto, Ontario
Normand Proulx, MD, FRCP(C)
Nephrologist
CISSS de L’Outaouais
Lecturer, McGill University
Gatineau, Quebec
Guidelines for the management of advanced chronic kidney disease (CKD), including dialysis, support intensive control of elevated phosphate levels due to a broad array of complications. These include increased risk of secondary hyperparathyroidism, cardiovascular (CV) events, and death. Despite guidelines, control of hyperphosphatemia in CKD has been consistently poor. In some studies, fewer than 30% of dialysis patients have been within the target range. Phosphate binders, along with diet, are a mainstay of therapeutic strategies. Simple dosing is relevant to patient care. Due to the complexity of CKD and its many comorbidities, phosphate binders with a low pill burden are an important variable for adherence and reaching treatment targets.
Background
As declining kidney function approaches and then falls below 30 mL/min/1.73 m2, the risk of hyperphosphatemia increases.4 One reason is that phosphate homeostasis depends on urinary excretion, a key function of the kidney.5 Hence, it follows that anuric patients on dialysis are at the greatest risk of hyperphosphatemia. In addition, the kidney participates in balancing parathyroid hormone (PTH), fibroblast growth factor (FGF23), vitamin D, and other biochemical factors that govern phosphorous metabolism.6 In defining the pathophysiology of hyperphosphatemia, the Kidney Disease Improving Global Outcomes (KDIGO) guidelines for CKD-related mineral and bone disorders emphasized the interrelationship of elevated phosphate, calcium, and PTH in CKD.1 Of several loops resulting in hyperphosphatemia, declining renal synthesis of vitamin D triggers a reduction in GI absorption of calcium which may in turn stimulate a greater production of PTH resulting in a greater release of phosphorus, from bone.5 However, once the eGFR falls below 20-25 mL/min/1.73 m2, phosphorus reabsorption is maximally suppressed, and urinary compensatory phosphate excretion may no longer keep up with phosphorus intake and release from bone, resulting in hyperphosphatemia.
For an exclusive interview with Dr. Robert Ting on the impact to clinical practice, click here
Diet and phosphate binding agents are cornerstones of hyperphosphatemia treatment, but management guidelines encourage attention to the interrelationship of other associated metabolic disturbances. While phosphate binding agents are required in almost all patients with advanced kidney disease to reach guideline targets, strategies to correct other metabolic imbalances, such as vitamin D, calcium and PTH levels, are often required.
Hyperphosphatemia can be associated with muscle cramps, tetany, perioral numbness or tingling, but the degree to which these symptoms are caused by elevated serum phosphate levels or by accompanying metabolic disturbances is unclear. Patients with hyperphosphatemia can also develop bone and joint pain, pruritis and rash. For diagnosis, routine monitoring of serum phosphate, as well as serum calcium, PTH, and alkaline phosphatase, is advised once patients have Stage 3a or higher CKD, according to KDIGO guidelines.1 Stage 3a CKD is defined as an estimated glomerular filtration (eGFR) rate of 45–59 mL/min/1.73 m2. By the time renal impairment reaches Stage 3b (eGFR 30–44 mL/min/1.73 m2) or greater severity, the guidelines recommend monitoring of serum phosphate and calcium at least every three months.
The normal serum phosphate level is variably defined.7 Ranges of 0.9 to 1.5 mmol/L are typical in healthy individuals, although retrospective analyses suggest risk of CKD progression is observed at levels lower than 1.5 mmol/L. In observational studies, increased concentrations of serum phosphate, which are frequently accompanied by elevations in PTH, FGF23, calcium, and calcium-phosphate product (CaxP), is associated with increased risk for valvular calcifications, left ventricular hypertrophy, heart failure, CV death, and all-cause death.8 Several studies with phosphate-binding therapy, including a systemic review and meta-analysis, have associated reductions in serum phosphate with improved survival.9-11 On the basis of the large body of data associating elevated serum phosphate levels with increased mortality and the retrospective evidence associating reductions in serum phosphate with a survival benefit, the target for serum phosphate is defined in the KDIGO guidelines as the “normal range.” Prospective randomized control trials verifying and quantifying the mortality benefit from treating hyperphosphatemia have yet to be completed but are now ongoing.
CV disease, which accounts for more than 50% of deaths in patients with CKD,12 is regarded as the most common cause of death related to elevated serum phosphate levels. Vascular calcification, which occurs in association with the interrelated metabolic disturbances that include hyperphosphatemia, is implicated in ischemic events. Vascular calcifications are also a suspected cause of impaired CV function that leads to heart failure, sudden cardiac death, and peripheral artery disease.11,13
There are mechanisms other than vascular calcification by which hyperphosphatemia directly or indirectly contributes to increased risk of morbidity and mortality. Due to its exacerbating effect on calcium metabolism, hyperphosphatemia either directly or indirectly increases the risk of bone pain and bone fracture related to impaired bone metabolism.4,11 It is also associated with debilitating pruritus,14 and it increases the risk of calciphylaxis, a rare but life-threatening complication.15 Secondary hyperparathyroidism, which is characterized by parathyroid gland hyperplasia, is a related but potentially independent contributor to risk of both CV and bone disease.16
Management
Most patients with advanced CKD and essentially all patients on dialysis have hyperphosphatemia.14 Due to the risks associated with elevated serum phosphate, the KDIGO guidelines recommend active interventions to lower phosphate toward normal ranges in patients with Stage 3a or greater CKD severity.1 Although phosphorus is the essential mineral employed by cells throughout the body, phosphate, which represents a binding of oxygen to phosphorus that is used by all biological systems, is the typical target of evaluation.6
There are currently 3 approaches to lowering phosphate levels in patients with CKD:
1. Dietary restriction of phosphate intake:
Active intervention includes dietary restriction of foods high in phosphate that may be derived from various sources. Organic phosphate is derived from either animal protein or plant based sources while inorganic phosphate is found in sodas and is used as an additive to prolong the shelf-life of packaged foods. Phosphate bioavailability in serum largely depends on the source with inorganic phosphate of packaged foods and sodas having the greatest bioavailability (80-100%3). Hence inorganic phosphate sources should be avoided in a patient with CKD. While the bioavailability of phosphorous in inorganic sources, such as prepared or packaged foods and soda is an estimated 80% to 100%,17 the bioavailability of phosphorus in plant sources, such as grains, legumes, or nuts, ranges from 20% to 40%.18,19 The bioavailability in animal protein, such as meat, fish, eggs, or milk, ranges from 40% to 60%.17 Nutritional guidelines issued by the National Kidney Foundation’s Kidney Disease Outcomes Quality initiative (KDOQI) include information on the identification of foods high in phosphates and strategies to design low-phosphate diets.20 Although diet can provide a meaningful reduction in serum phosphate levels, some studies indicate that less than half of patients remain adherent to diets low in phosphate.19 In patients on low phosphate diets, close monitoring of nutritional status is appropriate owing to the risk of inadequate proteins or other essential foods. Successful initiatives are likely to require education of both the patient and his or her family members, and nutritional advice tailored to the patient’s lifestyle and cultural background.19
2. Elimination of phosphate by dialysis
Phosphate is cleared by hemodialysis, although it is dependent on such variables as the flow rate and the characteristics of the dialyzer membrane. However, hemodialysis can only effectively remove phosphate from the serum during a typical 4 hours of dialysis treatment, which clears about 900 mg of phosphate.21 One explanation for this limited clearance is that most of the phosphate load in a CKD patient is found in the intracellular space rather than in the blood and the intra- to extracellular solute transfer rate is slow.22 Two studies have demonstrated that more phosphate can be removed with nocturnal dialysis sessions of longer duration.23,24 More typical dialysis sessions are helpful for reducing serum phosphate levels but they fall below typical dietary intake of phosphate, which occurs at a rate of 1000 mg per day even on low-phosphate diets. In more typical diets, the intake can be more than twice as high with absorption rates estimated at about 60%.25,26
3. Reduction of intestinal absorption of phosphate:
Due to the limits of diet and dialysis in lowering hyperphosphatemia, phosphate binders should be considered in most or all patients with late-stage CKD and remain a standard of care in patients on dialysis and peritoneal dialysis. In the GI tract, these phosphate binders exchange an anion of phosphate found in food with a cation, such as carbonate, acetate, or citrate, to form a nonabsorbable compound excreted in the feces.18 Hence the mechanism of action of binders explains why they must be taken with meals in order to be effective. Although the licensed binders are effective, they employ several different mechanisms. Differences between agents are potentially meaningful for defining relative efficacy and safety as well as practical considerations, particularly daily pill burden.
This latter issue is relevant to the adherence essential for sustained reductions in serum phosphate. Although pill burden is just one of several obstacles for patients remaining on long-term therapy, it is a fundamental step to the goal of lowering phosphate levels to reduce associated risks. In clinical studies, non-adherence to phosphate binders has ranged widely, but there are multiple studies to suggest that only about half of patients remain adherent over prolonged periods.27
Implementing a Comprehensive Approach to Treatment
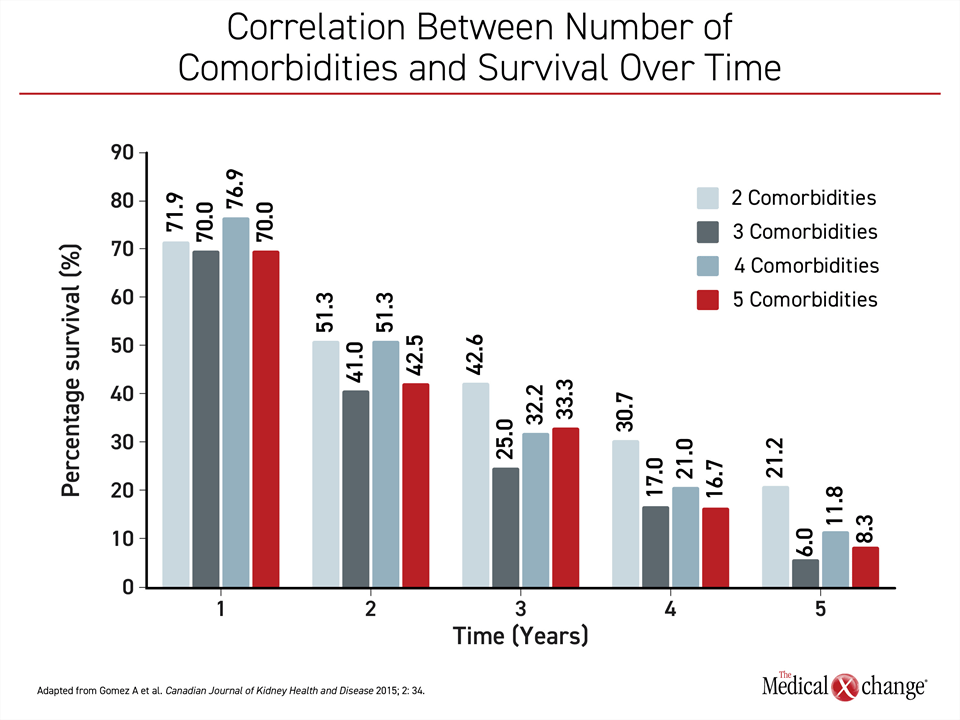
Hyperphosphatemia and CKD typically occur in the context of multiple morbidities, including diabetes mellitus, anemia, and CV disease or risk factors for CV disease, such as hypertension, all of which require treatment.28 One obstacle to adherence for pharmacologic control of hyperphosphatemia is the sheer number of pharmacologic therapies required in this complex population. Due to this complexity, there is growing interest in developing multidisciplinary teams that can address the multiple health issues faced by patients with advanced CKD or who have already progressed to dialysis. There is evidence this is effective. In a meta-analysis of 21 studies with more than 10,000 patients, multidisciplinary care models for CKD defined as teams comprised of nephrologists, cardiologists, pharmacists, and dieticians, were associated with slower rates of eGFR decline and lower rates of mortality.29 This type of comprehensive approach to treatment is justified. The strong correlation between number of comorbidities and survival over time supports an aggressive approach that includes optimal adherence to therapies that slow disease progression30 (Figure 1).
Phosphate Binders: Selecting the Appropriate Therapy
Phosphate binders are required in most patients with advanced CKD to improve serum phosphate levels. According to the KDIGO guidelines, the target is an acceptable range, defined as 1.13 – 1.78 mmol/L, although there is no clearly-defined level above which phosphate levels impose risks. So far, there is no prospective evidence of a mortality benefit for those reaching any given target level of serum phosphate, but two randomized control trials, HiLo and PHOSPHATE,31,32 are underway to address this question. Given the plausibility of benefit and the retrospective data supporting a reduction in mortality from treatment of elevated serum phosphate, phosphate binders remain a standard of care.
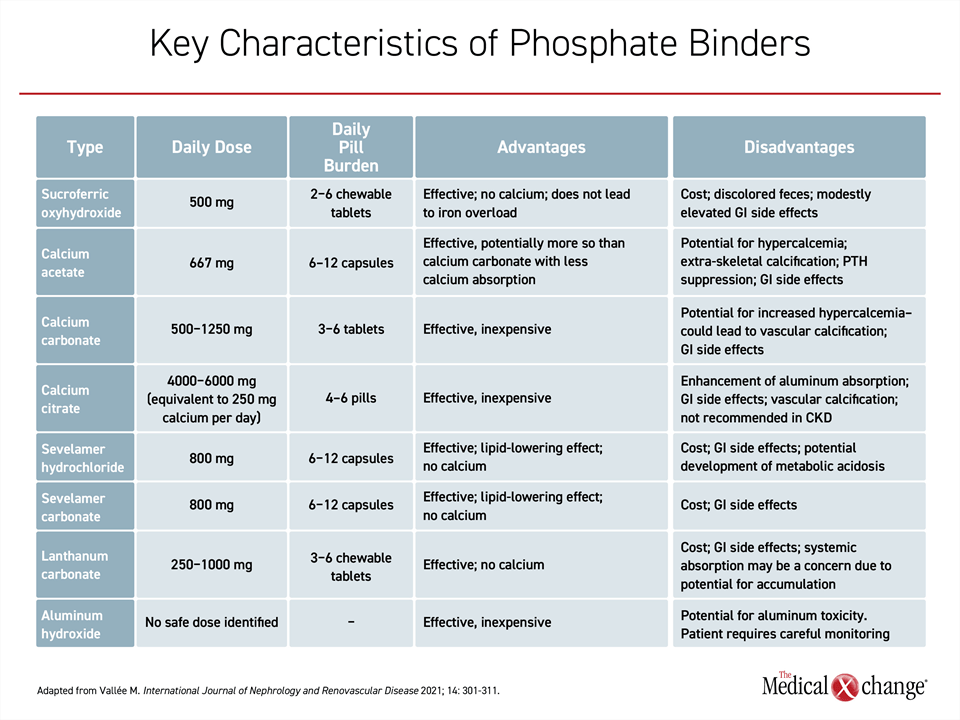
The introduction of phosphate binders into the management of advanced CKD is a relatively simple step, but these agents are not interchangeable on the basis of numerous potentially meaningful characteristics, including their mechanisms, their risk of adverse effects, their cost, and their pill burden. While aluminum hydroxide has largely fallen out of favor because of the risk of toxicity, there are three calcium-containing phosphate binders, two binders that include sevelamer, one that contains lanthanum, and sucroferric oxyhydroxide, a novel agent and the most recently approved (Table 1).
There are few controlled trials comparing these options. The calcium-based binders, calcium carbonate, calcium acetate, and calcium citrate, are widely used but not particularly good at binding phosphate and there has been concern raised by studies associating these binders with an increase in calcium load with progression of vascular calcification.33 The risk can be reduced by limiting the number of tablets used, but this will not permit the phosphorus targets to be achieved in most patients due to the reduced binding capacity.34
The two sevelamer-containing phosphate binders, sevelamer hydrochloride and sevelamer carbonate, are also commonly used. In controlled studies, these have been associated with sustained serum phosphate reductions in patients on dialysis or peritoneal dialysis. They are not associated with risk of elevated calcium.35 However, sevelamer has off-target effects on the GI tract that have included bleeding, nausea, and constipation.36 In some cases, such side effects as dysphagia and bowel obstruction have resulted in hospitalization and surgery.37 From a day-to-day patient perspective, the relative weak phosphate-binding capacity is likely to represent the biggest drawback. Both varieties of sevelamer require up to 12 pills per day to achieve treatment targets. In addition, sevelamer pills are big and must be swallowed whole, making the patient experience less enjoyable.
Lanthanum carbonate, like the sevelamer-containing drugs, does not contain calcium, but it is associated with side effects, including those involving the GI tract as well as muscle symptoms.38 In typical dosing, lanthanum carbonate requires only 3 to 6 pills per day to achieve target phosphate levels, but there is a potential for substantial accumulation of this binder in the bones.39 The clinical consequences of this accumulation are uncertain, but this feature, along with its cost, might explain its limited use. Lanthanum tablets cannot be swallowed whole to be effective and many patients must crush the tablet before taking the binder with a meal because of their hard consistency. Furthermore, palatability is less enjoyable because of the chalky texture.
Sucroferric oxyhydroxide, the phosphate binder most recently approved, is a chewable tablet taken three times per day, with meals. When chewed, patients often report a berry-like taste, making the experience more enjoyable than the other binders. It has also demonstrated efficacy in patients with end-stage renal disease, including those on dialysis. In a phase 3 trial that conducted a direct comparison to sevelamer hydrochloride, one of the most widely-prescribed phosphate binders, sucroferric oxyhydroxide was found to be non-inferior with regard to phosphate control. However, it did have advantages, including a much lower daily pill burden (3.1 chewable pills vs. 8.1 non-chewable tablets to achieve similar phosphate levels) and a more favorable safety profile.40 Specifically, while GI adverse events occurred in both study arms, they occurred at a lower rate on sucroferric oxyhydroxide (33.6% vs. 45.1%).
In another study, outcomes were compared among those maintained on sucroferric oxyhydroxide to those started on sucroferric oxyhydroxide but switched to another phosphate binder at 90 days.41 At 2 years, those on maintenance sucroferric oxyhydroxide were more likely to achieve a phosphate level of ≤1.78 mmol/L, had lower annual hospitalization rates, and took 50% fewer pills. In the recently published VERIFIE study, phosphorus serum levels fell from 2.03 to 1.71 mmol/L with sucroferric oxyhydroxide on an average daily dose of 2.3 pills.42 In addition, the proportion of patients with a serum phosphorus level <1.78 mmol/L climbed from 29.9% at study entry to 63.0% at the end of follow-up.
The most commonly reported GI side effect reported with sucroferric oxyhydroxide is loose stools. These are mild to moderate and tended to subside early in treatment without specific therapies or treatment changes.40 Clinical experience has led to the suggestion of initiating sucroferric oxyhydroxide at a lower dose of 500 mg daily to be chewed with the largest meal of the day in order to minimize the risk of experiencing loose stools. Thereafter the dose may be uptitrated by 500 mg (one pill) every 2-4 weeks until the target phosphate level is achieved. The maximum daily recommended dose is 3,000 mg (6 pills) per day. Due to the iron content of sucroferric oxyhydroxide stool often becomes dark, discoloured and patients should be advised of this. Of note, studies have reported only mild systemic absorption of iron with this binder.43,44
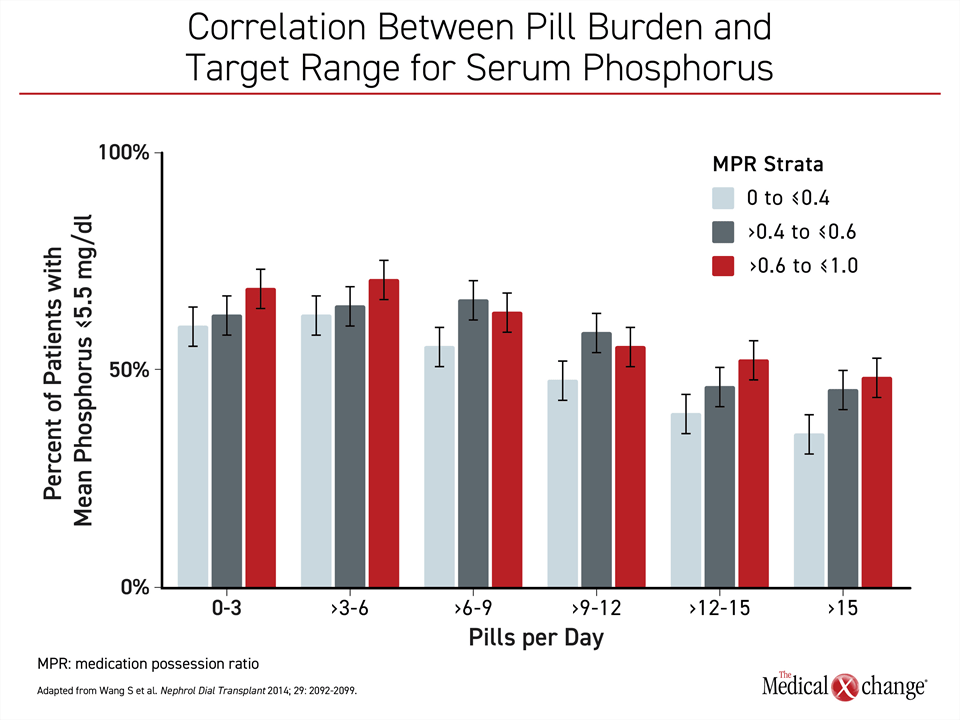
It has been estimated that phosphate binders represent about half of the pills required by dialysis patients.34 In a study evaluating the relationship between pill burden and adherence, a greater number of daily pills stratified by <3 pills, 3-6 pills, >12-15 pills, and >15 pills was associated with a stepwise increase in serum phosphorus levels.45 Conducted with 8,616 patients, the study also found a correlation between more pills and fewer patients in the target range for serum phosphorus (Figure 2).
There are numerous agents effective in binding phosphate for fecal excretion, but the options can be narrowed by treatment goals, including the avoidance of agents with the potential to elevate calcium and the selection of agents most likely to be compatible with sustained adherence. Relative tolerability, simplicity of dosing and potency are relative clinical advantages in general but have special relevance in patients with advancing CKD and multiple comorbidities.
Summary
The current guidelines for the management of hyperphosphatemia in advanced CKD have been derived from retrospective studies correlating elevated serum phosphate levels with increased risk of mortality. Further trials are now underway, but the available data associating lower serum phosphate levels with improved survival in CKD patients with hyperphosphatemia, particularly those on dialysis, are compelling. These data have provided the basis for current guidelines that call for serum phosphate targets within the normal range.
To achieve this target without exacerbating coexisting metabolic abnormalities, including the risk of calcium overload induced by calcium-based phosphate binders to which is linked an elevated risk of CV events, non-calcium-based phosphate binders are a cornerstone of hyperphosphatemia management. Of the multiple agents in different chemical classes available, most, but not all, impose a large pill burden, which is relevant to patient adherence. The simplest options require only three pills per day or less than half of the alternatives requiring the highest number of daily pills.
For optimal outcomes in patients with advanced CKD, a multidisciplinary team managing the multiple risks commonly shared in this population is guideline-recommended. Treatment of hyperphosphatemia cannot be isolated from other metabolic abnormalities encountered as renal function declines.
References
1. Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int 2017;92(1):26-36. DOI: 10.1016/j.kint.2017.04.006.
2. Farrand KF, Copley JB, Heise J, Fridman M, Keith MS, Poole L. Analysis of serum phosphate control and phosphate binder utilization in incident hemodialysis patients. Int J Nephrol Renovasc Dis 2014;7:261-9. DOI: 10.2147/IJNRD.S58037.
3. Young EW, Akiba T, Albert JM, et al. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2004;44(5 Suppl 2):34-8. DOI: 10.1053/j.ajkd.2004.08.009.
4. Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 2005;16(2):520-8. DOI: 10.1681/ASN.2004070602.
5. Cupisti A, Gallieni M. Urinary Phosphorus Excretion: Not What We Have Believed It to Be? Clin J Am Soc Nephrol 2018;13(7):973-974. DOI: 10.2215/CJN.06260518.
6. Rastogi A, Bhatt N, Rossetti S, Beto J. Management of Hyperphosphatemia in End-Stage Renal Disease: A New Paradigm. J Ren Nutr 2021;31(1):21-34. DOI: 10.1053/j.jrn.2020.02.003.
7. Chang WX, Xu N, Kumagai T, et al. The Impact of Normal Range of Serum Phosphorus on the Incidence of End-Stage Renal Disease by A Propensity Score Analysis. PLoS One 2016;11(4):e0154469. DOI: 10.1371/journal.pone.0154469.
8. Da J, Xie X, Wolf M, et al. Serum Phosphorus and Progression of CKD and Mortality: A Meta-analysis of Cohort Studies. Am J Kidney Dis 2015;66(2):258-65. DOI: 10.1053/j.ajkd.2015.01.009.
9. Jamal SA, Vandermeer B, Raggi P, et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet 2013;382(9900):1268-77. DOI: 10.1016/S0140-6736(13)60897-1.
10. Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008;52(3):519-30. DOI: 10.1053/j.ajkd.2008.03.020.
11. Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004;15(8):2208-18. DOI: 10.1097/01.ASN.0000133041.27682.A2.
12. Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol 2004;15(12):2959-64. DOI: 10.1097/01.ASN.0000145894.57533.C4.
13. Askar AM. Hyperphosphatemia. The hidden killer in chronic kidney disease. Saudi Med J 2015;36(1):13-9. DOI: 10.15537/smj.2015.1.9843.
14. Germain MJ. Uremic Pruritus: An Itch with Ominous Consequences. Am J Nephrol 2017;46(6):448-449. DOI: 10.1159/000484572.
15. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 2015;66(1):133-46. DOI: 10.1053/j.ajkd.2015.01.034.
16. Friedman EA. Consequences and management of hyperphosphatemia in patients with renal insufficiency. Kidney Int Suppl 2005(95):S1-7. DOI: 10.1111/j.1523-1755.2005.09500.x.
17. Calvo MS, Moshfegh AJ, Tucker KL. Assessing the health impact of phosphorus in the food supply: issues and considerations. Adv Nutr 2014;5(1):104-13. DOI: 10.3945/an.113.004861.
18. Barreto FC, Barreto DV, Massy ZA, Drueke TB. Strategies for Phosphate Control in Patients With CKD. Kidney Int Rep 2019;4(8):1043-1056. DOI: 10.1016/j.ekir.2019.06.002.
19. Umeukeje EM, Mixon AS, Cavanaugh KL. Phosphate-control adherence in hemodialysis patients: current perspectives. Patient Prefer Adherence 2018;12:1175-1191. DOI: 10.2147/PPA.S145648.
20. Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am J Kidney Dis 2020;76(3 Suppl 1):S1-S107. DOI: 10.1053/j.ajkd.2020.05.006.
21. Waheed AA, Pedraza F, Lenz O, Isakova T. Phosphate control in end-stage renal disease: barriers and opportunities. Nephrol Dial Transplant 2013;28(12):2961-8. DOI: 10.1093/ndt/gft244.
22. Achinger SG, Ayus JC. The role of daily dialysis in the control of hyperphosphatemia. Kidney Int Suppl 2005(95):S28-32. DOI: 10.1111/j.1523-1755.2005.09504.x.
23. Pierratos A, Ouwendyk M, Francoeur R, et al. Nocturnal hemodialysis: three-year experience. J Am Soc Nephrol 1998;9(5):859-68. DOI: 10.1681/ASN.V95859.
24. Mucsi I, Hercz G, Uldall R, Ouwendyk M, Francoeur R, Pierratos A. Control of serum phosphate without any phosphate binders in patients treated with nocturnal hemodialysis. Kidney Int 1998;53(5):1399-404. DOI: 10.1046/j.1523-1755.1998.00875.x.
25. Gutzwiller JP, Schneditz D, Huber AR, Schindler C, Gutzwiller F, Zehnder CE. Estimating phosphate removal in haemodialysis: an additional tool to quantify dialysis dose. Nephrol Dial Transplant 2002;17(6):1037-44. DOI: 10.1093/ndt/17.6.1037.
26. Kalantar-Zadeh K. Patient education for phosphorus management in chronic kidney disease. Patient Prefer Adherence 2013;7:379-90. DOI: 10.2147/PPA.S43486.
27. Arenas MD, Malek T, Gil MT, Moledous A, Alvarez-Ude F, Reig-Ferrer A. Challenge of phosphorus control in hemodialysis patients: a problem of adherence? J Nephrol 2010;23(5):525-34. (https://www.ncbi.nlm.nih.gov/pubmed/20119931).
28. Vadakedath S, Kandi V. Dialysis: A Review of the Mechanisms Underlying Complications in the Management of Chronic Renal Failure. Cureus 2017;9(8):e1603. DOI: 10.7759/cureus.1603.
29. Shi Y, Xiong J, Chen Y, et al. The effectiveness of multidisciplinary care models for patients with chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol 2018;50(2):301-312. DOI: 10.1007/s11255-017-1679-7.
30. Gomez AT, Kiberd BA, Royston JP, et al. Comorbidity burden at dialysis initiation and mortality: A cohort study. Can J Kidney Health Dis 2015;2:34. DOI: 10.1186/s40697-015-0068-3.
31. Edmonston DL, Isakova T, Dember LM, et al. Design and Rationale of HiLo: A Pragmatic, Randomized Trial of Phosphate Management for Patients Receiving Maintenance Hemodialysis. Am J Kidney Dis 2021;77(6):920-930 e1. DOI: 10.1053/j.ajkd.2020.10.008.
32. clinicaltrials.gov. Pragmatic randomized trial of high or standard PHosphAte Targets in End-stage Kidney Disease (PHOSPHATE).
33. London GM, Marchais SJ, Guerin AP, Boutouyrie P, Metivier F, de Vernejoul MC. Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol 2008;19(9):1827-35. DOI: 10.1681/ASN.2007050622.
34. Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol 2009;4(6):1089-96. DOI: 10.2215/CJN.00290109.
35. Chertow GM, Burke SK, Raggi P, Treat to Goal Working G. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 2002;62(1):245-52. DOI: 10.1046/j.1523-1755.2002.00434.x.
36. Madan P, Bhayana S, Chandra P, Hughes JI. Lower gastrointestinal bleeding: association with Sevelamer use. World J Gastroenterol 2008;14(16):2615-6. DOI: 10.3748/wjg.14.2615.
37. Vallee M, Weinstein J, Battistella M, Papineau R, Moseley D, Wong G. Multidisciplinary Perspectives of Current Approaches and Clinical Gaps in the Management of Hyperphosphatemia. Int J Nephrol Renovasc Dis 2021;14:301-311. DOI: 10.2147/IJNRD.S318593.
38. Zhang C, Wen J, Li Z, Fan J. Efficacy and safety of lanthanum carbonate on chronic kidney disease-mineral and bone disorder in dialysis patients: a systematic review. BMC Nephrol 2013;14:226. DOI: 10.1186/1471-2369-14-226.
39. Spasovski GB, Sikole A, Gelev S, et al. Evolution of bone and plasma concentration of lanthanum in dialysis patients before, during 1 year of treatment with lanthanum carbonate and after 2 years of follow-up. Nephrol Dial Transplant 2006;21(8):2217-24. DOI: 10.1093/ndt/gfl146.
40. Floege J, Covic AC, Ketteler M, et al. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int 2014;86(3):638-47. DOI: 10.1038/ki.2014.58.
41. Coyne DW, Ficociello LH, Parameswaran V, et al. Sucroferric Oxyhydroxide in Maintenance Hemodialysis: A Retrospective, Comparative Cohort Study. Kidney Med 2020;2(3):307-316. DOI: 10.1016/j.xkme.2020.01.009.
42. Vervloet MG, Boletis IN, de Francisco ALM, et al. Real-world safety and effectiveness of sucroferric oxyhydroxide for treatment of hyperphosphataemia in dialysis patients: a prospective observational study. Clin Kidney J 2021;14(7):1770-1779. DOI: 10.1093/ckj/sfaa211.
43. Covic AC, Floege J, Ketteler M, et al. Iron-related parameters in dialysis patients treated with sucroferric oxyhydroxide. Nephrol Dial Transplant 2017;32(8):1330-1338. DOI: 10.1093/ndt/gfw242.
44. Sprague SM, Floege J. Sucroferric oxyhydroxide for the treatment of hyperphosphatemia. Expert Opin Pharmacother 2018;19(10):1137-1148. DOI: 10.1080/14656566.2018.1491548.
45. Wang S, Alfieri T, Ramakrishnan K, Braunhofer P, Newsome BA. Serum phosphorus levels and pill burden are inversely associated with adherence in patients on hemodialysis. Nephrol Dial Transplant 2014;29(11):2092-9. DOI: 10.1093/ndt/gft280.