Nephrology
Toronto Complement Conference (TCC) 2024
Emerging Treatment Options for Unmet Need in Complement 3 Glomerulopathy
Toronto – Complement 3 glomerulopathy (C3G) is a rare, progressive kidney disease caused by dysregulation of the alternative complement pathway. No treatments targeting the underlying cause of the disease are currently available, but new agents addressing the alternative complement pathway are now in clinical trials. First presented earlier this year, results from two major Phase 3 trials were placed in context at the TCC. In each trial, a novel agent in development for C3G was well-tolerated and associated with substantial reductions in proteinuria and with stabilization of kidney function. Longer-term data, as demonstrated in APPEAR-C3G 12-month results, show promising safety and long-term outcomes, and offer new hope for a challenging disorder.
The Alternative Complement Pathway and Current Treatment Options
With an estimated incidence of 1 to 2 cases per million per year, C3G affects patients of all ages but is often first diagnosed in children and young adults. Symptoms include proteinuria and hematuria, and reduced kidney function. The prognosis is generally poor, with 30% to 50% of affected patients progressing to kidney failure within 10 years, noted Dr. Anne-Laure Lapeyraque, Chief of Pediatric Nephrology, Sainte Justine University Hospital Center, Montreal, Quebec.
Current treatment recommendations are based on international consensus and include supportive therapy with renin-angiotensin-aldosterone system blockers, lipid control, and a low-salt diet. Otherwise, “treatment is, still today, based on very unspecific immunosuppression,” stated Dr. Christoph Licht, Professor, Department of Pediatrics, University of Toronto and Head, Division of Nephrology, Hospital for Sick Children, Toronto. He further explained, “We have a very small subgroup of patients spontaneously recovering, but after that, patients who progress and show severe disease presentation are subjected to prednisone and MMF [mycophenolate mofetil] treatment.”
For an exclusive interview with Dr. Christoph Licht on the impact to clinical practice, click here
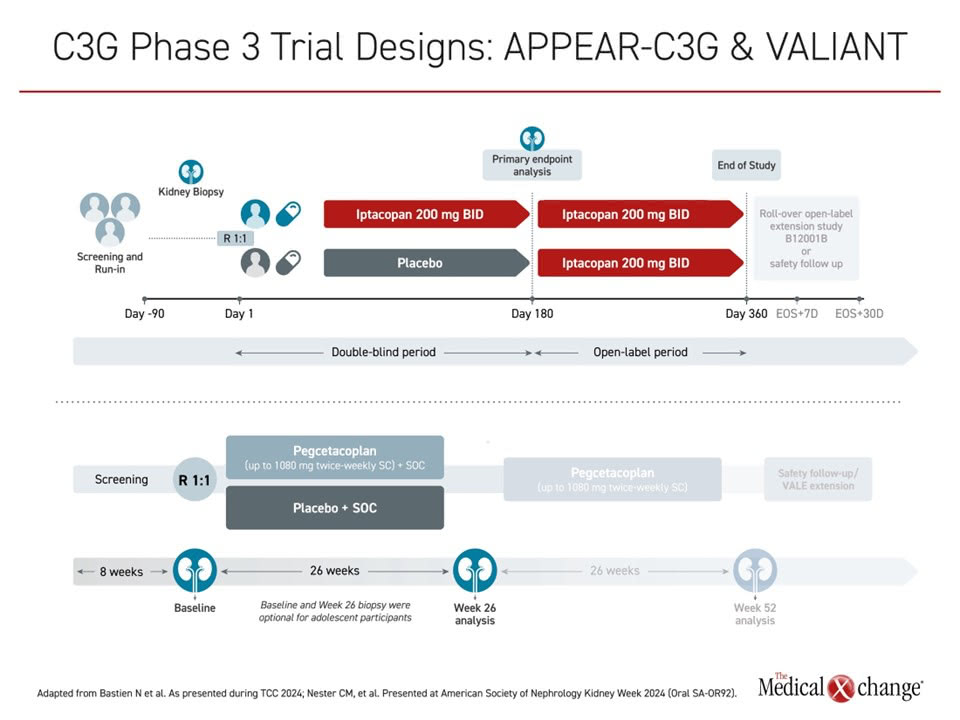
More specific treatment with complement blockers is currently only considered as rescue therapy for those who don’t achieve complete remission with immunosuppression, and in the context of clinical trials. C3G involves dysregulation at the level of C3 convertase, “so we need agents that address that part of the cascade, and that is what we have now,” Dr. Licht said. “We’re just at the end of phase 3 clinical trial testing, and the results are quite encouraging.” Results from the two major and recent trials to address C3 convertase, APPEAR-C3G with 12-month data and VALIANT with 26-week data, were both positive when initially presented earlier this year (Figure 1).
“C3G involves dysregulation at the level of C3 convertase, ‘so we need agents that address that part of the cascade, and that is what we have now’”
APPEAR-C3G Study: Oral Proximal Complement Inhibitor
APPEAR-C3G was a pivotal Phase 3 trial of iptacopan, an oral proximal complement inhibitor that specifically binds Factor B and inhibits the alternative pathway. Iptacopan was approved by the FDA in 2023 and the EMA in 2024 for the treatment of another rare disorder, paroxysmal nocturnal hemoglobinuria. It is also being investigated in a number of other rare diseases, Dr. Lapeyraque explained.
In this double-blind, placebo-controlled study, 74 adult patients with C3G were randomly assigned to receive twice-daily iptacopan (200 mg) or placebo on top of supportive care. Patients were included if they had a biopsy within 12 months, with a reduced C3 level and a urine protein-to-creatinine ratio (UPCR) ≥1 g/g. The double-blind treatment phase was 6 months, followed by open-label treatment for all patients for an additional 6 months.
The primary endpoint for the double-blind phase was reduction in proteinuria from baseline, measured by 24-hour UPCR. Of note, baseline demographics showed patients in the iptacopan arm appeared to have a more severe phenotype at baseline vs. the placebo group, Dr. Lapeyraque pointed out.
Six- and 12-Month Data Show Sustained Results and Favourable Safety Profile
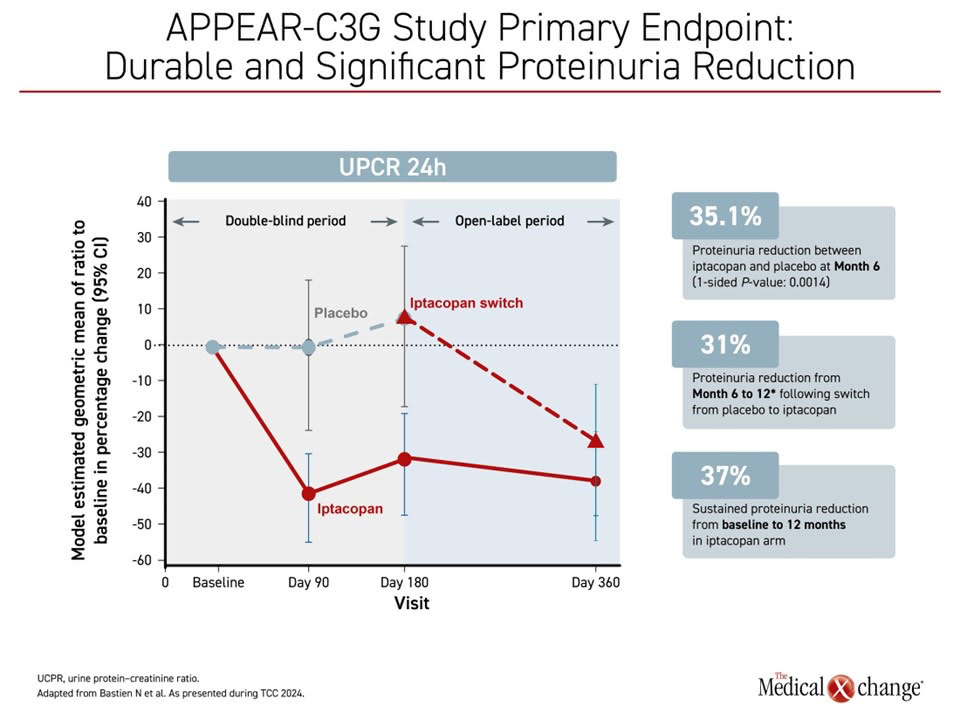
Primary outcome 6-month results, presented previously at the ERA 2024, showed the change from baseline in 24-hour UPCR was a reduction of 30.2% vs. an increase of 7.6% in the placebo group, a relative reduction of 35.1% and a statistically significant outcome (1-sided P=0.0014).
Further, the significant reduction was sustained up to 12 months through the open-label period. For the group initially treated with placebo, there was a 31% reduction in proteinuria from month 6 to month 12, and a sustained proteinuria reduction out to 12 months in the group initially treated with iptacopan at 37% from baseline (Figure 2).
There was no significant difference between treatment and placebo groups on the secondary endpoint of change from baseline in glomerular inflammation on the histological total activity score at month 6, however, an exploratory analysis looking at the circulating biomarker levels showed inhibition of the alternative complement pathway by iptacopan treatment.
At 12 months, iptacopan stabilized estimated glomerular filtration rate (eGFR) in the group previously on placebo, and maintained stable eGFR in the group initially treated with iptacopan (+0.84 mL/min/1.73m2).
Reviewing these data, Dr. Lapeyraque reported that treatment was well tolerated, with a favourable safety profile. There were no deaths, cases of meningitis and/or meningococcal sepsis, or discontinuations due to treatment-emergent adverse events (TEAEs) with iptacopan treatment. Most adverse events were mild-to-moderate in severity.
Long-term Efficacy and Safety in Phase 2, 33-month Extension Study
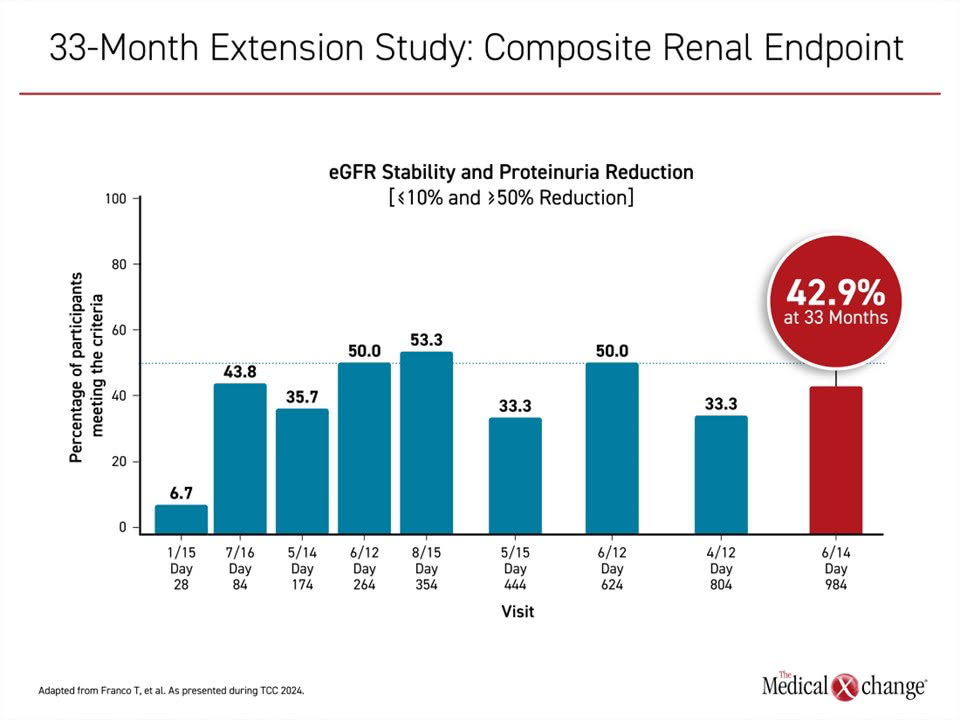
In a separate analysis presented at TCC 2024, researchers reported updated data from a 33-month extension study of C3G patients enrolled in a Phase 2 trial of iptacopan. Of 27 patients in the original study, 26 entered the extension study and 22 completed the last visit at 33 months.
Researchers reported long-term treatment with iptacopan was associated with sustained reductions in proteinuria and preservation of eGFR in these patients (Figure 3). The improvements were associated with “substantial” reduction in the alternative complement pathway, linked to normalization of serum C3. No new safety findings were seen over the long term, including among patients with disease recurrence after a kidney transplant on triple immunosuppression. This long-term data with the oral proximal complement inhibitor iptacopan shows promise towards the future treatment of patients with C3G.
VALIANT Trial: Phase 3 Data at Up to 26 Weeks
The second recent trial reviewed at this year’s TCC was a phase 3 investigation of pegcetacoplan, a C3/C3b inhibitor that blocks formation of C3 convertase. The VALIANT trial included 124 patients with a confirmed histologic diagnosis of C3G or primary immune complex-mediated membranoproliferative glomerulonephritis (IC-MPGN), with or without previous renal transplant, with proteinuria >1 g/day and an eGFR >30 mL/min/1.73m2, treated with a stable standard of care regimen, and with evidence of active renal disease on biopsy.
Patients were randomly assigned to treatment with pegcetacoplan at a dose of up to 1080 mg twice-weekly given by subcutaneous injection, or placebo, and followed up to 26 weeks. There is a planned extension period of 26 weeks, as well as safety follow-up beyond that in the VALE extension study.
The primary endpoint was the log-transformed ratio of the UPCR at 26 weeks vs. baseline. The results, first presented recently at ASN Kidney Week 2024, showed a statistically significant relative reduction of 68.3% in pegcetacoplan vs. placebo (P<0.0001) The reductions were seen across all subgroups including age, disease type and transplant status.
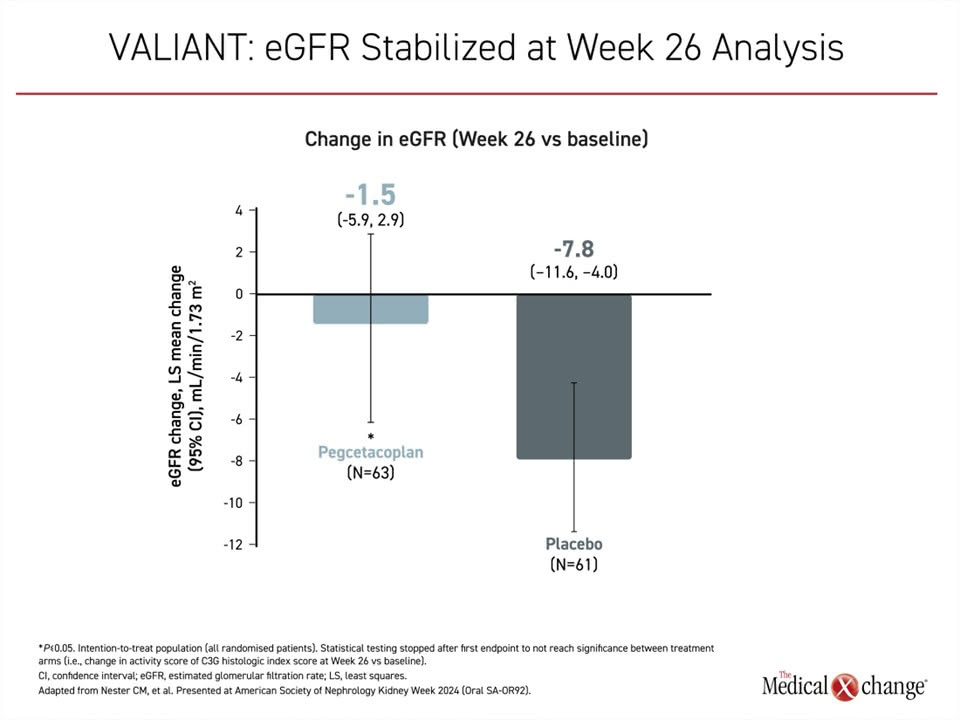
Treated patients were significantly more likely to have a >50% reduction in proteinuria, with 60.3% of patients vs. 4.9% of patients on placebo (P<0.001) meeting this endpoint. Pegcetacoplan significantly stabilized eGFR compared with placebo, with a change in eGFR from baseline of a 1.5 mL/min/1.73m2 reduction vs. 7.8 mL/min/1.73m2 in the placebo group (P=0.03) (Figure 4).
Treatment was also well tolerated. TEAE severity and frequency were similar between groups. There were four serious infections, three in the pegcetacoplan group and one in the placebo group. There was one death in the treated group, from COVID-19 pneumonia, considered unrelated to treatment. There were no cases of encapsulated N. meningitidis.
Newer Therapies Might Be More Relevant to Modifiable Disease Mechanisms
Dr. Lapeyraque put study results into perspective by pointing out that both agents provided a significant reduction in proteinuria, preservation of eGFR over time, a decrease in C3/C3b deposits in the glomeruli, and significant inhibition of the alternative complement pathway on C3 and sC5b-9 measurements. Both were considered well-tolerated. However, while APPEAR-C3G and Phase 2 extension study results confirmed efficacy and safety with iptacopan over 12 months and 33 months, respectively, 12-month data for the planned VALIANT extension are not yet available for pegcetacoplan.
Both of these therapies are designed to address the underlying cause of C3G, which involves dysregulation of the alternative complement pathway, presented Dr. Licht. Activation of C3 by the convertase C3bBb turns it into C3b, which is then further processed into an inactive form, forming split products. Produced in excess, split products are deposited in the glomerular filter, triggering the phenotype of C3G.
One of the two causes involves antibodies forming to components or regulators of C3bBb. This is an autoimmune process, but mutations in genes related to components of these same cascades can also initiate the same cascade and clinical consequences, he continued.
There is considerable interest and optimism about the potential for more effective control of C3G if these therapies are further validated and meet regulatory approval. Addressing the complement pathway rather than symptoms alone, it is hoped that tighter control of the underlying pathology will meet the current unmet need for this disease.
“There is considerable interest and optimism about the potential for more effective control of C3G if these therapies are further validated and meet regulatory approval.”
New Therapies on Their Way into Clinical Practice: Other Considerations
There are no direct comparisons of iptacopan, for which there are now median 12-month follow-up data, and pegcetacoplan, for which there are now 26-week data, but the agents might not be interchangeable. In particular, iptacopan is taken orally twice daily whereas pegcetacoplan requires twice-weekly subcutaneous injections after initial administration in the clinic. This may prove an important consideration for patients, should either be fully approved for the treatment of C3G.
There continues to be important research activity in this area, Dr. Lapeyraque explained. Other potential studies may determine whether it is possible to block both the C3/C3 convertase axis and the C5/C5 convertase axis at the same time, which might avoid any potentially problematic imbalance between the two. She discussed another investigational drug, KP104, an anticomplement therapy in development for C3G, which was developed with this idea in mind. It is an investigational agent currently in Phase 2 trials in both IgA nephropathy and C3G.
Conclusion
New and promising anti-complement agents targeting the underlying cause of C3G are now in clinical trials. Aiming to meet the critical unmet need in this rare and progressive kidney disease, both oral and injectable proximal complement inhibitors are being examined for their ability to correct the dysregulation of the alternative complement pathway. These trials have demonstrated significant improvements in key clinical outcomes, including proteinuria reductions and eGFR stabilization, marking an important step forward in addressing disease progression. Confirming the long-term safety and efficacy of these agents will be critical to their successful integration into treatment paradigms. With continued progress, these innovative therapies hold the potential to transform care and outcomes for patients living with C3G.