Expert Review
Proper Use of Anticoagulants for Stroke Prevention in Atrial Fibrillation
Chapter 3: Practical Considerations for Oral Anticoagulants in Stroke Prevention
Ashkan Shoamanesh, MD
Associate Professor, Division of Neurology
McMaster University
Hamilton, Ontario
Non-vitamin K antagonist oral anticoagulants (NOACs) are often not employed in recommended or adequate doses for stroke prevention in patients with atrial fibrillation (AF). Largely attributed to fear among clinicians of inducing bleeding events, this suggests there is an incomplete understanding of the major opportunity these agents provide for risk reduction. In almost all patients, benefit-to-risk ratio from stroke prevention is favorable. When compared to warfarin, the four available NOACs have demonstrated similar or superior efficacy in pivotal trials. The modest differences amongst the NOACs in safety relative to warfarin and in pharmacokinetics relative to each other are relevant to dosing or choice of NOAC in some patients. Recognizing the importance of anticoagulation in AF patients and the principles of appropriate therapy provides an important opportunity to reduce an important source of morbidity and mortality in Canada.
Simplifying Choices of NOACs in Stroke Prevention
Anticoagulation is an effective means of reducing the risk posed by AF for stroke and other thromboembolic events, but clot inhibition raises the risk of bleeding. For individuals with AF where anticoagulation is indicated for stroke prevention, the benefit of reducing potentially disabling or lethal ischemic strokes typically exceeds the risk of intracranial hemorrhage (ICH) or major bleeding by a significant margin. Clinicians must weigh these competing outcomes to avoid permitting fear of an iatrogenic ICH or a serious bleed to obscure the more important opportunity for preventing stroke with appropriate doses of anticoagulation.
In calculating these risks, a focus solely on bleeding scores such as the HAS-BLED or HEMORR2HAGES scales provides an incomplete picture, as these scales tend to be co-linear with thromboembolic risk scores (CHADS2 or CHA2DS2-VASc) and the absolute risk of ischemic events. Typically, the margins of benefit from anticoagulation outweigh the absolute increased risk of hemorrhagic events in patients deemed at high risk for bleeding. Regrettably, national registries continue to show alarming rates of anticoagulation under usage. In the PINNACLE registry, a quality initiative sponsored by the American College of Cardiology, only 45% of patients with AF without apparent contraindications to anticoagulation were receiving appropriate anticoagulation.1
The risk of strokes associated with AF, like risk of AF itself, increases with advancing age.2,3 Although the risk of major bleeding, including ICH, also increases with age, stroke prevention retains a favorable benefit-to-risk ratio in AF patients across age groups.4 In a retrospective study that looked specifically at AF patients 90 years or older, there was a net clinical benefit whether the oral anticoagulant warfarin was compared to no treatment or to aspirin.5 When NOACs were compared to warfarin in this age group, the newer agents were associated with a lower risk of ICH, resulting in an increased net clinical advantage in this very elderly population.
The major guidelines do not impose any age restrictions on stroke prevention in AF patients. Yet, there are multiple sets of data indicating that oral anticoagulation is either withheld or provided at inappropriately low doses in older individuals otherwise indicated for this therapy.6,7 In one study, the proportion of AF patients receiving an oral anticoagulant at discharge fell incrementally with each decade of age, declining from 75% among those less than 70 years of age to 24% among those 90 years of age or older.8 When prescribing clinicians were asked why anticoagulation was withheld, risk of hemorrhage was prominent among cited justifications.
Yet, due to greater risk posed by AF-related stroke relative to major bleeding for disability and mortality, the relative advantage of oral anticoagulation is at least as great or greater in older relative to younger individuals.9 In the major clinical trials of oral anticoagulation for AF patients, the net benefit, which is the reduction in stroke after accounting for any increased risk of bleeding, is derived from full therapeutic doses.10 With only a limited number of exceptions, such as in patients with severely impaired renal function who were not eligible for the pivotal AF randomized trials, the guideline-recommended doses are appropriate in all patients at risk of AF-associated stroke.
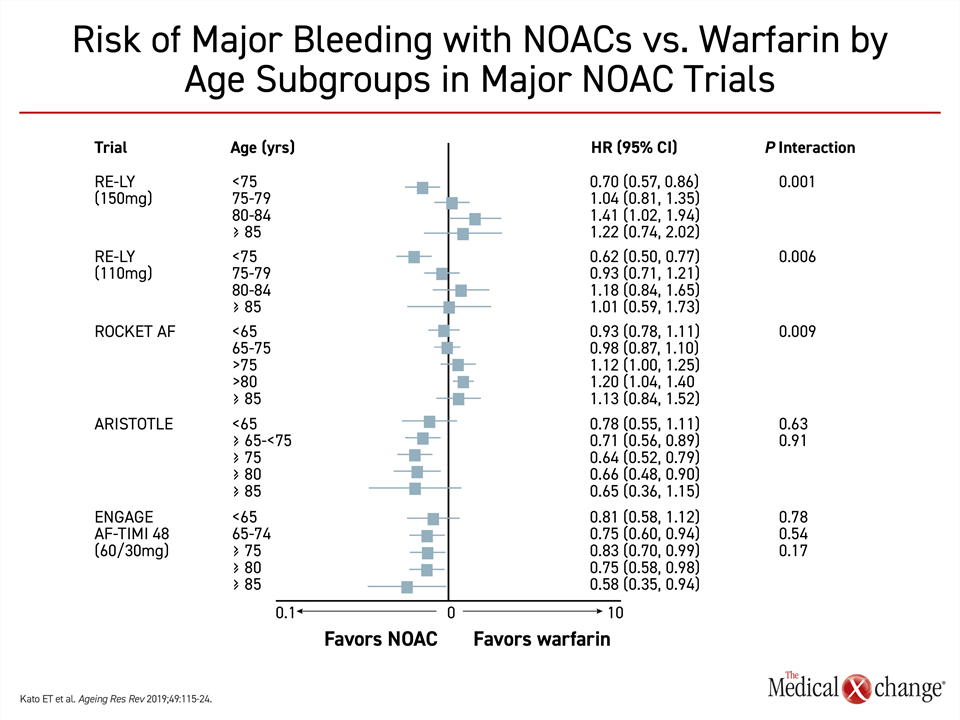
Warfarin is an acceptable oral anticoagulant for stroke prevention in patients with AF, but NOACS are the preferred oral agents in major guidelines on the basis of greater convenience, similar or superior stroke prevention, a halving of ICH risk, and similar or lower risk of major bleeding.11-13 There is evidence that the potential safety advantage of NOACs increases with advancing age. In the ARISTOTLE trial with apixaban and the ENGAGE AF-TIMI 48 trial with edoxaban,14,15 bleeding rates trended lower with NOACs relative to warfarin with each 5-year increment of patient age10 (Fig. 1). In a prespecified analysis of the ENGAGE AF-TIMI trial that explored this relationship, the reduction in risk of major bleeding translated into a greater net clinical benefit for those 75 years and older relative to those younger.16
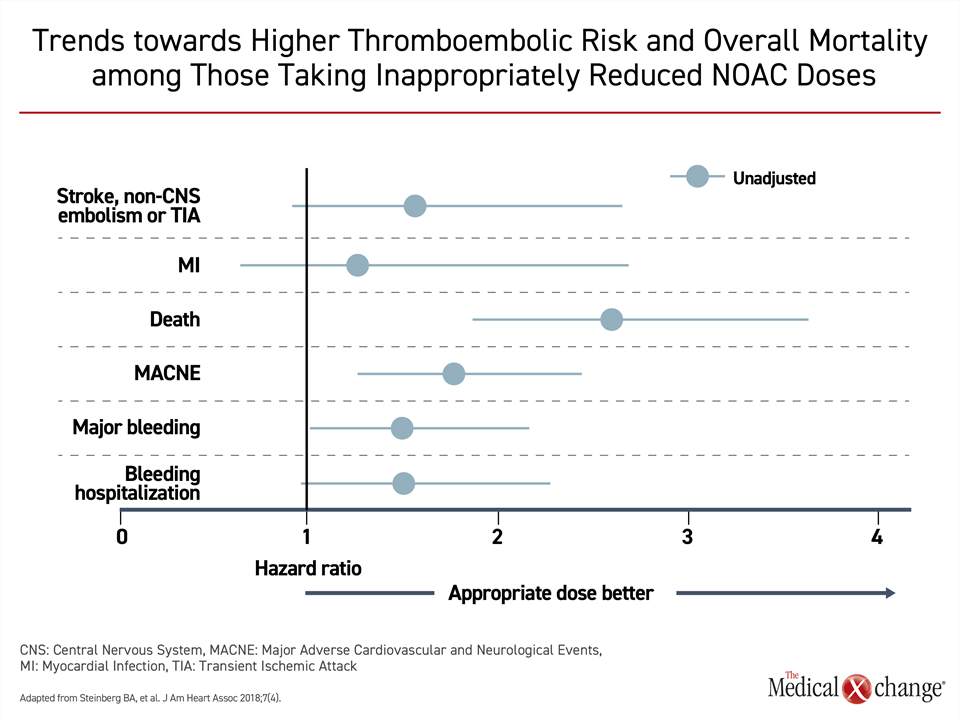
The evidence that older AF patients typically derive greater net clinical benefit from oral anticoagulants relative to younger patients does not negate efforts to minimize bleeding risk at any age. It is, for example, appropriate to consider and address modifiable risk factors, such as, inadequately controlled hypertension, excess alcohol consumption, propensity toward falling, and unnecessary use of medications associated with increased bleeding risk, as well as identifying and treating sources of anemia.13 However, the presence of risk factors for bleeding, like older age, should not preclude full dose oral anticoagulation in AF patients who are candidates for stroke prevention. In ORBIT-II, 1289 (16%) of 7,925 patients evaluated received NOAC doses judged to be inappropriately reduced.17 When this group was compared to those receiving standard doses, the risk of death was highly significantly increased (Fig. 2).
The misperception that age and vulnerability to bleeding should alter strategies for stroke prevention is common. Numerous observational studies have documented high rates of no or inadequate oral anticoagulation in AF patients who are older, have comorbidities, or are otherwise thought to be at increased risk of bleeding.18 In GARFIELD-AF, history of bleeding was a predictor of inadequate anticoagulation in AF patients.19 The data from the GARFIELD-AF registry are among those that support the guidelines. In those who were candidates for anticoagulation, the risk of major bleeding was lower rather than higher when anticoagulation was withheld (0.5% vs. 0.8%; P<0.001; perhaps due to confounding by indication rather than a treatment effect), while the risk of stroke (1.6% vs. 1.1%; P<0.001) and risk of all-cause mortality (5.3% vs. 3.9%; P<0.001) were significantly greater. In a case-control multicenter study inappropriate and off label reduced dosing of NOACs was one of the most potent risk factors for stroke, leading to an adjusted ~3.5-fold increased risk.20 Inappropriate reduced dosing of NOACs seems to only increase the risk of thromboembolic events, without any benefits in reducing hemorrhagic events.21
Similarly, there have been concerns regarding anticoagulation in AF patients with ischemic stroke who are noted to have evidence of occult microbleeds, defined as round intraparenchymal lesions of hemosiderin staining that are <10 mm in diameter, on blood sensitive magnetic resonance imaging sequences. Microbleeds are most often markers of age-related cerebral small vessel disease in AF patients, notably either hypertensive arteriopathy or cerebral amyloid angiopathy. Microbleeds are associated with a heightened risk of future incident ICH in this context, but also are at heightened risk for ischemic stroke.22 Consistent with the theme however, the absolute rates of ischemic stroke far overshadow that of ICH in ischemic stroke patients with microbleeds on MRI, irrespective of microbleed severity, and anticoagulant therapy should not be withheld in these patients when indicated.22,23
Among AF patients over the age of 65 with an additional risk factor for stroke, such as hypertension or diabetes, there are only a few situations in which benefit remains unclear. One is a history of ICH, as these patients have been excluded from AF anticoagulation randomized trials. Even within this high-risk subgroup, meta-analyses of observational data suggest reduced all-cause mortality and net benefit with anticoagulation, including in patients with lobar intracerebral hemorrhage that have the highest rate of long-term ICH recurrence.24,25 Interestingly, the risk of ischemic stroke is reported to be as high as 10 to 13% per year in patients with previous intracerebral hemorrhage who do not receive anticoagulation.26,27
In an algorithm proposed by a recent review, NOACs were recommended four or more weeks after imaging has confirmed resolution of ICH despite the absence of a controlled trial.28 In the Canadian-led NASPAF-ICH trial (unpublished), which randomized 30 AF patients with previous intracerebral hemorrhage to standard dosing NOAC therapy or aspirin 81 mg daily, there was only one ischemic stroke over mean follow-up of 1.53 years that occurred in an aspirin assigned participant. There was no recurrent intracerebral hemorrhage in either arm of the study. All participants had close home blood pressure monitoring to ensure target <130/80 mm Hg, which is an essential prerequisite—that can halve the risk of intracerebral hemorrhage recurrence—when considering anticoagulation (re-)initiation in this population.
These preliminary results are being investigated further in ongoing randomized trials. The largest, ENRICH-AF, is a global phase 3 randomized trial, where AF patients with a CHA2DS2-VASc ≥2 who have had a previous ICH will be randomized to edoxaban or a control strategy.30 Controls will receive no antithrombotic therapy or antiplatelet monotherapy at the discretion of the local investigator. The study dose of edoxaban is 60 mg, but a dose of 30 mg will be used in those meeting criteria for the lower dose, consistent with the ENGAGE-AF TIMI 48 and current on-label dosing criteria. The co-primary endpoints of ischemic stroke, hemorrhagic and undetermined stroke will be evaluated once 123 primary events have accrued. A composite of ischemic events, including myocardial infarction and all-cause death, are among secondary endpoints. The study, which will also monitor ICH and major hemorrhage, is intended to determine whether NOAC for stroke prevention provides a net clinical benefit in this high-risk AF population.
Not All NOACs Are Alike: Differentiating Characteristics
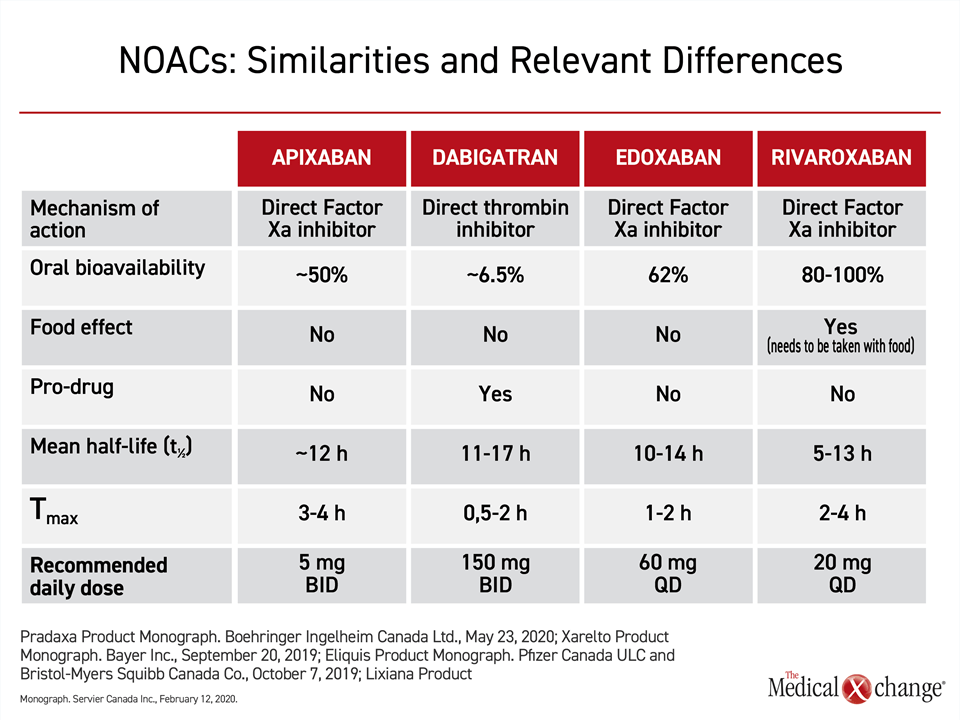
In current guidelines, including those in Canada, the four NOACs approved for prevention of stroke in patients with AF are recommended without preference.11-13 In pivotal phase 3 trials, each has demonstrated comparable or greater efficacy than warfarin in stroke prevention and similar or lower risk of bleeding.14,15,31,32 Relative to warfarin, all are considered more convenient in major guidelines because they can be administered in a fixed dose without therapeutic monitoring.12,13,33 The specific pharmacokinetic properties of NOACs, although similar, range to a degree that might be clinically relevant for some patients (Table 1).34
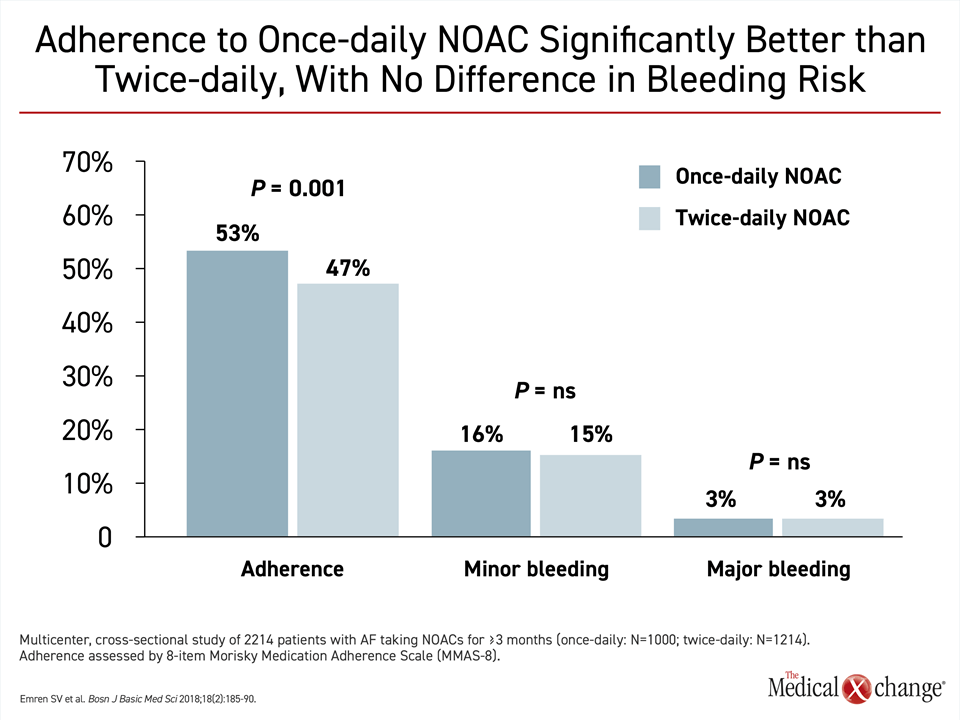
Of these differences, frequency of dosing, relative risk of drug-drug interactions, risk of a food effect on drug metabolism, and dependence on renal clearance are among characteristics most likely to be clinically relevant for clinicians or patients attempting to select among the available NOACs. Dosing frequency might be relevant to patient preference and to long-term efficacy. Not all patients might perceive a once-daily dose more convenient, but there is evidence that the simpler daily regimen provides a modest but significant improvement in adherence (Fig. 3).
Only one NOAC, rivaroxaban, is associated with a food effect. According to prescribing guidelines, the once-daily rivaroxaban—at doses greater than 10 mg daily that are used in AF—should be taken with a meal in order to achieve optimal bioavailability.35 Pharmacokinetics (PK) analyses drawn from studies in patients and healthy volunteers suggest that adequate drug concentrations over a 24-hour period are achieved whether once-daily rivaroxaban is taken with the evening or the morning meals, but it is important to take the drug at the same meal each day to sustain 24-hour protection. All the NOACs, apart for dabigatran, which is supplied as a capsule containing tartaric acid that is essential for its gastrointestinal absorption, can be crushed for oral use in patients with dysphagia or administration via feeding tubes.
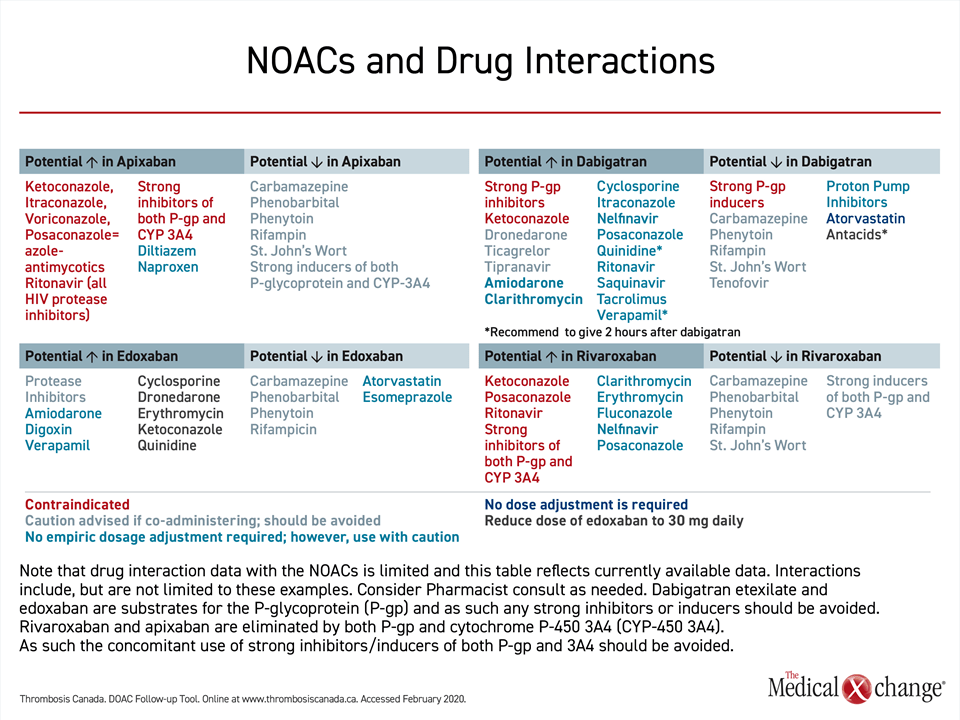
None of the NOACs are free of the risk of a drug-drug interaction, but the risks differ. Apixaban and rivaroxaban compete with drugs or foods that are metabolized by the hepatic cytochrome P450 3A4 isoenzyme. Examples include some antifungal agents, some tyrosine kinase inhibitors, and grapefruit juice. These two NOACs, which are moderately dependent on liver metabolism for elimination, should therefore be used cautiously in patients taking CYP 3A4 inhibitors or inducers. Dabigatran and edoxaban are not dependent or have minimal dependence on liver metabolism.
All four drugs employ the P-glycoprotein (P-gp) transport system, creating the potential for drug-drug interactions with strong P-gp inhibitors or inducers, such as cyclosporine, digoxin or certain antiepileptic medications, particularly in patients with impaired renal clearance where the P-gp system is most active. However, labeling restrictions are based largely on pivotal trial designs. Earlier NOAC trials (apixaban, rivaroxaban and dabigatran) excluded concomitant use of strong P-gp inhibitors, producing a relative contraindication for these drugs in the presence of such medications (Table 2). However, in the ENGAGE-AF TIMI 48 trial the use of a strong P-gp inhibitor was listed as an indication for edoxaban dose adjustment, rather than an exclusion criterion. This trial was accordingly able to demonstrate the safety of the lower 30 mg edoxaban dose among patients on a strong P-gp inhibitor, and current drug labeling allows its use in this context.
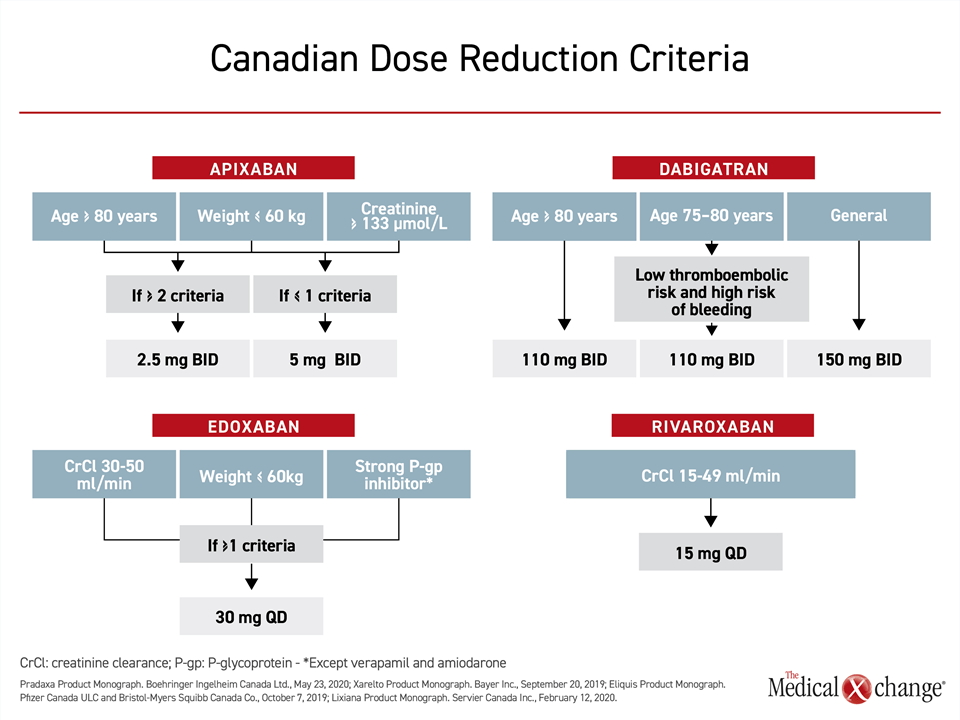
In most patients with renal impairment, NOACs remain a preferred option to warfarin if doses are adjusted appropriately.36 In the labeling, these adjustments are variably recommended by creatinine clearance, creatinine level, by weight, and by age, which is an important risk factor for impaired renal function in AF37 (Fig. 4). Dabigatran, at 85%, has the highest dependence of renal clearance. Apixaban, at approximately 27%, has the lowest. Edoxaban (50%) and rivaroxaban (36%) have a moderate dependence.
Evidence-Based Approach to AF-Associated Stroke Prevention
The major guidelines for prevention of stroke in patients with AF are relatively consistent, but there is evidence that more education of healthcare providers is needed. In a survey of healthcare providers in Canada, only 60% reported that they were comfortable prescribing oral anticoagulants.38 Although the majority of respondents recognized that dose adjustments based on renal function and age are appropriate, only about 25% recognized that rivaroxaban had a food effect. Studies that have shown high rates of inappropriately withheld anticoagulants or anticoagulants prescribed as suboptimal doses in AF patients who meet guideline-recommended criteria for stroke prevention reinforce the persistent knowledge gaps for an important approach to reduction of preventable stroke-related morbidity and mortality.10,39
The guidelines for primary or secondary stroke prevention in AF patients are not complicated. With few exceptions, most AF patients over the age of 65 years and many AF patients who are younger with additional vascular risk factors are candidates for oral anticoagulation. If all AF patients with an indication for oral anticoagulation were treated, and at appropriate doses, most AF-associated strokes would be prevented.20
In the guidelines, the appropriate use of oral anticoagulants for those with renal impairment, those schedule for surgery, and those recovering from a first stroke are detailed but straightforward. Although dose reductions are required in selected cases to maintain an optimal benefit-to-risk ratio, the prevalent inappropriate use of off-label lower doses of NOACs is emerging as a significant modifiable risk factor for AF-related stroke in current practice.
Summary
A more rigorous and uniform application of oral anticoagulation therapies in AF patients will reduce mortality and morbidity in Canada. All of the NOACs, although not necessarily interchangeable, have shown efficacy that is as good or superior to warfarin in clinical trials. The risk of ICH was halved and other forms of major bleeding were the same or lower. These therapies are relatively simple to employ. While it is important to recognize when dose adjustments are appropriate, the vast majority of AF patients who are candidates for stroke prevention should be on these treatments indefinitely at dosages proven to be therapeutic that are on label and adjusted appropriately to patient individual characteristics.
References
- Hsu JC, Maddox TM, Kennedy KF, et al. Oral Anticoagulant Therapy Prescription in Patients With Atrial Fibrillation Across the Spectrum of Stroke Risk: Insights From the NCDR PINNACLE Registry. JAMA Cardiol 2016;1:55-62.
- Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245-54.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119-25.
- van Walraven C, Hart RG, Connolly S, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation: the atrial fibrillation investigators. Stroke 2009;40:1410-6.
- Fordyce CB, Hellkamp AS, Lokhnygina Y, et al. On-Treatment Outcomes in Patients With Worsening Renal Function With Rivaroxaban Compared With Warfarin: Insights From ROCKET AF. Circulation 2016;134:37-47.
- Fang MC, Stafford RS, Ruskin JN, Singer DE. National trends in antiarrhythmic and antithrombotic medication use in atrial fibrillation. Arch Intern Med 2004;164:55-60.
- Nieuwlaat R, Capucci A, Camm AJ, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J 2005;26:2422-34.
- Hylek EM, D’Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke 2006;37:1075-80.
- Marinigh R, Lip GY, Fiotti N, Giansante C, Lane DA. Age as a risk factor for stroke in atrial fibrillation patients: implications for thromboprophylaxis. J Am Coll Cardiol 2010;56:827-37.
- Kato ET, Goto S, Giugliano RP. Overview of oral antithrombotic treatment in elderly patients with atrial fibrillation. Ageing Res Rev 2019;49:115-24.
- Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453-68.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125-e51.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893-962.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104.
- Kato ET, Giugliano RP, Ruff CT, et al. Efficacy and Safety of Edoxaban in Elderly Patients With Atrial Fibrillation in the ENGAGE AF-TIMI 48 Trial. J Am Heart Assoc 2016;5.
- Steinberg BA, Shrader P, Pieper K, et al. Frequency and Outcomes of Reduced Dose Non-Vitamin K Antagonist Anticoagulants: Results From ORBIT-AF II (The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II). J Am Heart Assoc 2018;7.
- Seelig J, Pisters R, Hemels ME, Huisman MV, Ten Cate H, Alings M. When to withhold oral anticoagulation in atrial fibrillation – an overview of frequent clinical discussion topics. Vasc Health Risk Manag 2019;15:399-408.
- Bassand JP, Accetta G, Al Mahmeed W, et al. Risk factors for death, stroke, and bleeding in 28,628 patients from the GARFIELD-AF registry: Rationale for comprehensive management of atrial fibrillation. PLoS One 2018;13:e0191592.
- Paciaroni M, Agnelli G, Caso V, et al. Causes and Risk Factors of Cerebral Ischemic Events in Patients With Atrial Fibrillation Treated With Non-Vitamin K Antagonist Oral Anticoagulants for Stroke Prevention. Stroke 2019;50:2168-74.
- Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-Vitamin K Antagonist Oral Anticoagulant Dosing in Patients With Atrial Fibrillation and Renal Dysfunction. J Am Coll Cardiol 2017;69:2779-90.
- Wilson D, Ambler G, Lee KJ, et al. Cerebral microbleeds and stroke risk after ischaemic stroke or transient ischaemic attack: a pooled analysis of individual patient data from cohort studies. Lancet Neurol 2019;18:653-65.
- Shoamanesh A, Charidimou A, Sharma M, Hart RG. Should Patients With Ischemic Stroke or Transient Ischemic Attack With Atrial Fibrillation and Microbleeds Be Anticoagulated? Stroke 2017;48:3408-12.
- Biffi A, Kuramatsu JB, Leasure A, et al. Oral Anticoagulation and Functional Outcome after Intracerebral Hemorrhage. Ann Neurol 2017;82:755-65.
- Murthy SB, Gupta A, Merkler AE, et al. Restarting Anticoagulant Therapy After Intracranial Hemorrhage: A Systematic Review and Meta-Analysis. Stroke 2017;48:1594-600.
- Nielsen PB, Larsen TB, Skjoth F, Gorst-Rasmussen A, Rasmussen LH, Lip GY. Restarting Anticoagulant Treatment After Intracranial Hemorrhage in Patients With Atrial Fibrillation and the Impact on Recurrent Stroke, Mortality, and Bleeding: A Nationwide Cohort Study. Circulation 2015;132:517-25.
- Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 2015;313:824-36.
- Hawkes MA, Rabinstein AA. Anticoagulation for atrial fibrillation after intracranial hemorrhage: A systematic review. Neurol Clin Pract 2018;8:48-57.
- Shoamanesh A, Charidimou A, Sheth KN. Comorbid Atrial Fibrillation in Cerebral Amyloid Angiopathy-related Intracerebral Hemorrhage: Between a Rock and a Hard Place. J Stroke Cerebrovasc Dis 2019;28:104351.
- clinicaltrials.gov. Edoxaban for intracranial hemorrhage survivors with atrial fibrillation (ENRICH-AF). https://clinicaltrialsgov/ct2/show/NCT03950076. Accessed June 15, 2020.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51.
- Andrade JG, Verma A, Mitchell LB, et al. 2018 Focused Update of the Canadian Cardiovascular Society Guidelines for the Management of Atrial Fibrillation. Can J Cardiol 2018;34:1371-92.
- Yeh CH, Hogg K, Weitz JI. Overview of the new oral anticoagulants: opportunities and challenges. Arterioscler Thromb Vasc Biol 2015;35:1056-65.
- Zhang L, Peters G, Haskell L, Patel P, Nandy P, Moore KT. A Cross-Study Analysis Evaluating the Effects of Food on the Pharmacokinetics of Rivaroxaban in Clinical Studies. J Clin Pharmacol 2017;57:1607-15.
- Harel Z, Sholzberg M, Shah PS, et al. Comparisons between novel oral anticoagulants and vitamin K antagonists in patients with CKD. J Am Soc Nephrol 2014;25:431-42.
- Kulkarni N, Gukathasan N, Sartori S, Baber U. Chronic Kidney Disease and Atrial Fibrillation: A Contemporary Overview. J Atr Fibrillation 2012;5:448.
- Piran S, Schulman S, Panju M, Pai M. Oral anticoagulant dosing, administration, and storage: a cross-sectional survey of Canadian health care providers. J Thromb Thrombolysis 2018;45:180-5.
- Fernandes L, Sargento-Freitas J, Milner J, et al. Ischemic stroke in patients previously anticoagulated for non-valvular atrial fibrillation: Why does it happen? Rev Port Cardiol 2019;38:117-24.
Chapter 3: Practical Considerations for Oral Anticoagulants in Stroke Prevention
Non-vitamin K antagonist oral anticoagulants (NOACs) are often not employed in recommended or adequate doses for stroke prevention in patients with atrial fibrillation (AF). Largely attributed to fear among clinicians of inducing bleeding events, this suggests there is an incomplete understanding of the major opportunity these agents provide for risk reduction. In almost all patients, benefit-to-risk ratio from stroke prevention is favorable. When compared to warfarin, the four available NOACs have demonstrated similar or superior efficacy in pivotal trials. The modest differences amongst the NOACs in safety relative to warfarin and in pharmacokinetics relative to each other are relevant to dosing or choice of NOAC in some patients. Recognizing the importance of anticoagulation in AF patients and the principles of appropriate therapy provides an important opportunity to reduce an important source of morbidity and mortality in Canada.
Show review