Expert Review
IAS 2011: Expert Review
Protease Inhibitors after 15 Years: Report from the IAS
Dr. Jean-Guy Baril
Clinique Médicale du Quartier Latin, Montreal, QC
Dr. Jason Brunetta
Maple Leaf Medical Clinic, Toronto, ON
Rome – In 1996, a protease inhibitor (PI)-based antiretroviral combination led to the first opportunity for long-term survival for most patients living with human immunodeficiency virus (HIV). On the 15th anniversary of that breakthrough, PIs are no longer a mandatory component of an effective antiretroviral regimen, but they remain an important and reliable option in HIV control. As they are among the most potent antiretroviral agents, PIs remain a reasonable choice in a wide variety of clinical scenarios, including first-line treatment. Although some of the initially introduced PIs remain available, the common PIs in most formularies are ritonavir-boosted atazanavir (ATV/r), darunavir (DRV/r), and lopinavir (LPV/r). Relative to first generation PIs, these have few dosing restrictions, a low pill burden, and less relative risk of adverse events. Despite the broad experience with all of these agents, optimal use of PIs remains a dynamic exploration of relative strengths and weaknesses, particularly in the context of an aging HIV-infected population. This update assembles key studies on PIs from the 2011 IAS meeting.
Protease Inhibitors Compared: Renal Function
Protease inhibitors (PIs) are potent and versatile antiretroviral agents that were the foundation of the first effective combination therapies for suppression of human immunodeficiency virus (HIV). While they continue to be used in a traditional three-drug regimen with two nucleoside reverse transcriptase inhibitors (NRTIs), they are being increasingly used in novel strategies, particularly in combinations that permit sparing of NRTIs which is an appealing approach to the reducing risk of adverse events and to simplify therapy. NRTI-sparing regimens may play an important role in reducing co-morbidities common to aging HIV patients, such as renal impairment. In traditional or novel applications, the current efforts to compare the relative characteristics of the most frequently prescribed PIs focus almost entirely on relative safety. In fact, one of the areas of greatest focus at this year’s IAS conference was relative effects on renal function, a rapidly-emerging concern in countries, including Canada, where the average age of individuals infected with HIV is rising rapidly.
Data indicated that PIs are associated with modest renal toxicity, but that risk may not be shared equally among agents within this class.
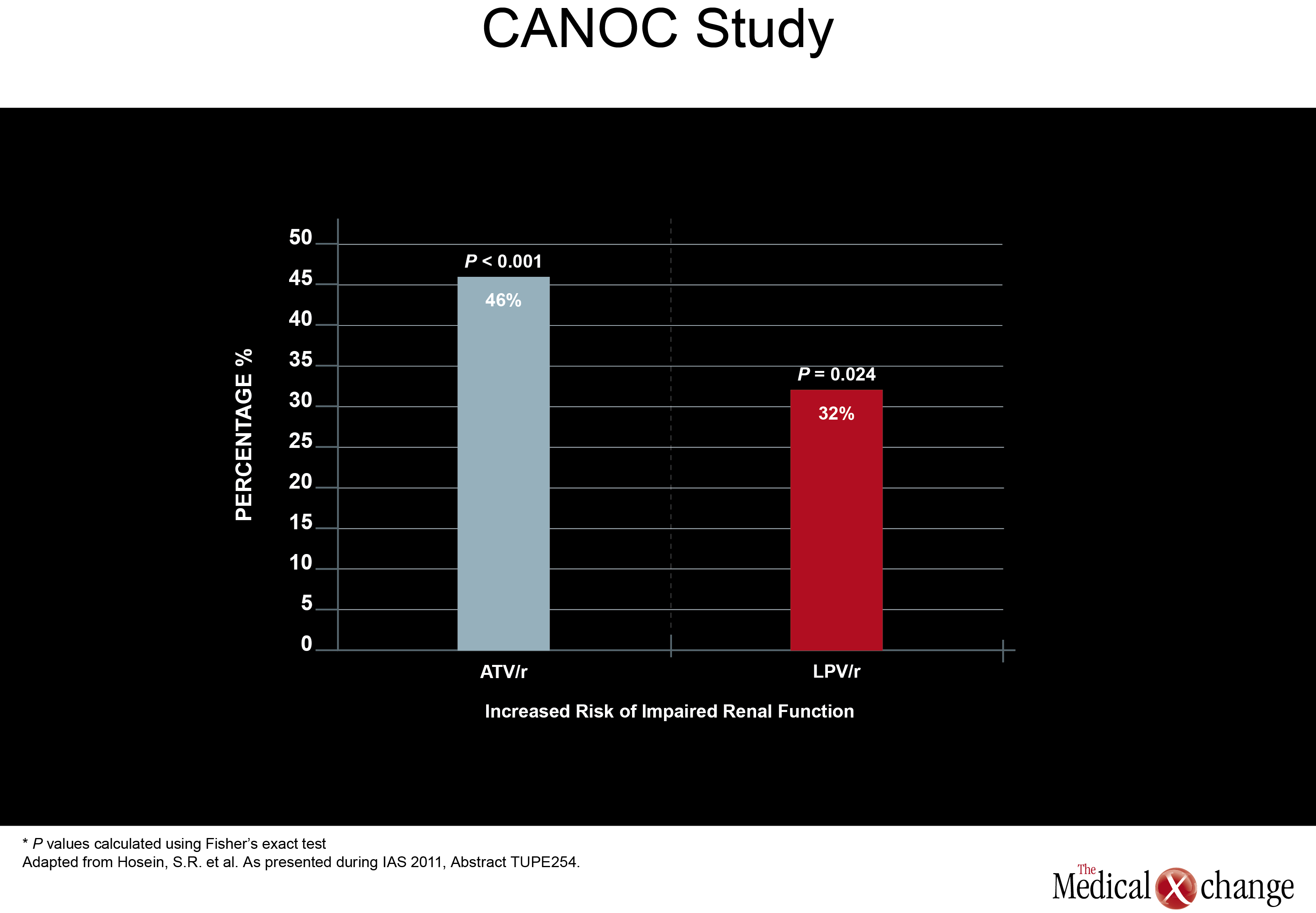
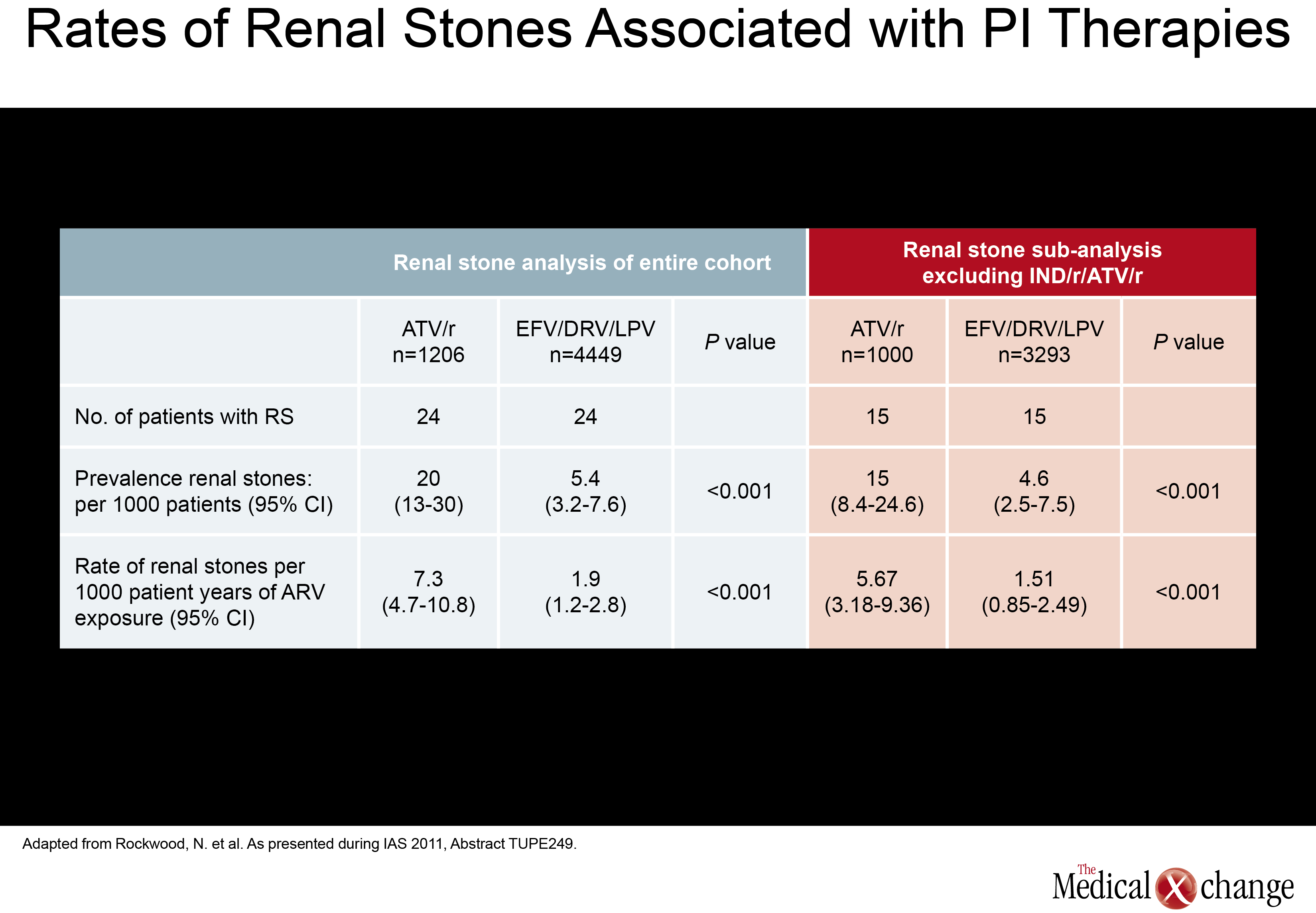
Of a set of new studies evaluating renal toxicity with PIs, one came from Canada. Drawn from the Canadian Observational Cohort Collaboration (CANOC), the data indicated that atazanavir (ATV) and lopinavir (LPV) were associated with modest renal toxicity compared to non-PI regimens. In this study, factors associated with impaired estimated glomerular filtration rate (eGFR) were assessed in 985 patients being followed at eight collaborating centres in British Columbia, Ontario and Quebec. Of the two PIs evaluated, ATV was associated with a hazard ratio (HR) of 1.46 (P<0.001) while LPV posed a HR of 1.32 (P=0.024) in a multivariate analysis when both were compared to antiretroviral therapy without a PI (Fig. 1). The authors, led by Sean R. Hosein of Canadian AIDS Treatment Information Exchange (CATIE), in Toronto, Ontario, suggested that the PI data are consistent with previous studies, including a cross-sectional analysis of the French Aquitaine cohort (Dauchy FA et al. Kidney Int 2011;80(3):302-9) and the collaborative EuroSIDA cohort study (Mocroft A et al. AIDS 2010; 24(11):1667-78). In a multivariate analysis conducted in the Aquitaine study, the odds ratio (OR) for developing proximal renal tubular dysfunctionon ATV was 1.28 per year of exposure. The OR for the same outcome for tenofovir (TDF) in the study was 1.23 per-year of exposure. In the EuroSIDA cohort, the OR among PIs for two consecutive measures of abnormal eGFR (<60 ml/min per 1.73 m2) was 1.21 for ATV (P=0.0003), 1.12 for indinavir (IDV) (P<0.0001), and 1.08 for LPV/r (P=0.03). In a separate study at the IAS meeting, the same issue was addressed when electronic medical records were reviewed for 2,115 patients starting antiretroviral therapy with efavirenz (EFV), ATV/r, LPV/r, or DVR/r over a recent nearly four-year period. The study was designed to identify the rate of renal toxicity, defined as eGFR <60 ml/min per 1.732. All participants had an eGFR >60ml/min at baseline. Compared to EFV, the HR elevation for renal impairment was not statistically significant for darunavir (DRV) (P=0.108), but was statistically significant for LPV (P=0.017) and ATV (P=0.004) after adjusting for gender, age, baseline eGFR, baseline CD4 count, hepatitis B and C status, prior exposure to TDF and IDV. The authors, led by Dr. Neesha Rockwood, Chelsea and Westminster Hospital, London, UK, noted that 50% of those who developed renal impairment did show recovery over follow-up, but they suggested PIs may not be interchangeable for this risk. The mechanism of potential kidney toxicity of ATV and LPV is not clear. Of course, caution is required when interpreting the results of retrospective cohort studies. In particular, patients in non-randomized studies may have received different prescriptions based on baseline eGFR. Therefore, any associations observed in retrospective analyses are best investigated in prospective, randomized, and controlled trials. New information about renal stones, another potential complication of antiretroviral therapy, was also presented at the IAS meeting. In this study, also led by Dr. Rockwood of London’s Chelsea and Westminster Hospital, investigators systematically looked for renal stones in patients taking ATV, LPV, DRV, or the non-nucleoside reverse transcriptase inhibitor (NNRTI) EFV. The rate of stones per 1000 patient years of exposure on ATV was 7.3, or more than three times higher, than the 1.9 rate associated with any of the other agents evaluated (P<0.001). The relative increase in risk remained similar after excluding patients previously exposed to IDV. This led the authors to conclude that ATV exposure is associated with significantly increased rate of renal stones when compared to the other therapies evaluated (EFV, LPV/r, and DPV) with and without adjusting for prior IDVexposure (Table 1).
PI Risks and Potency
Although renal impairment is a CV risk factor, it is well recognized that aging HIV patients should be closely monitored for all CV risk factors, regardless of regimen.
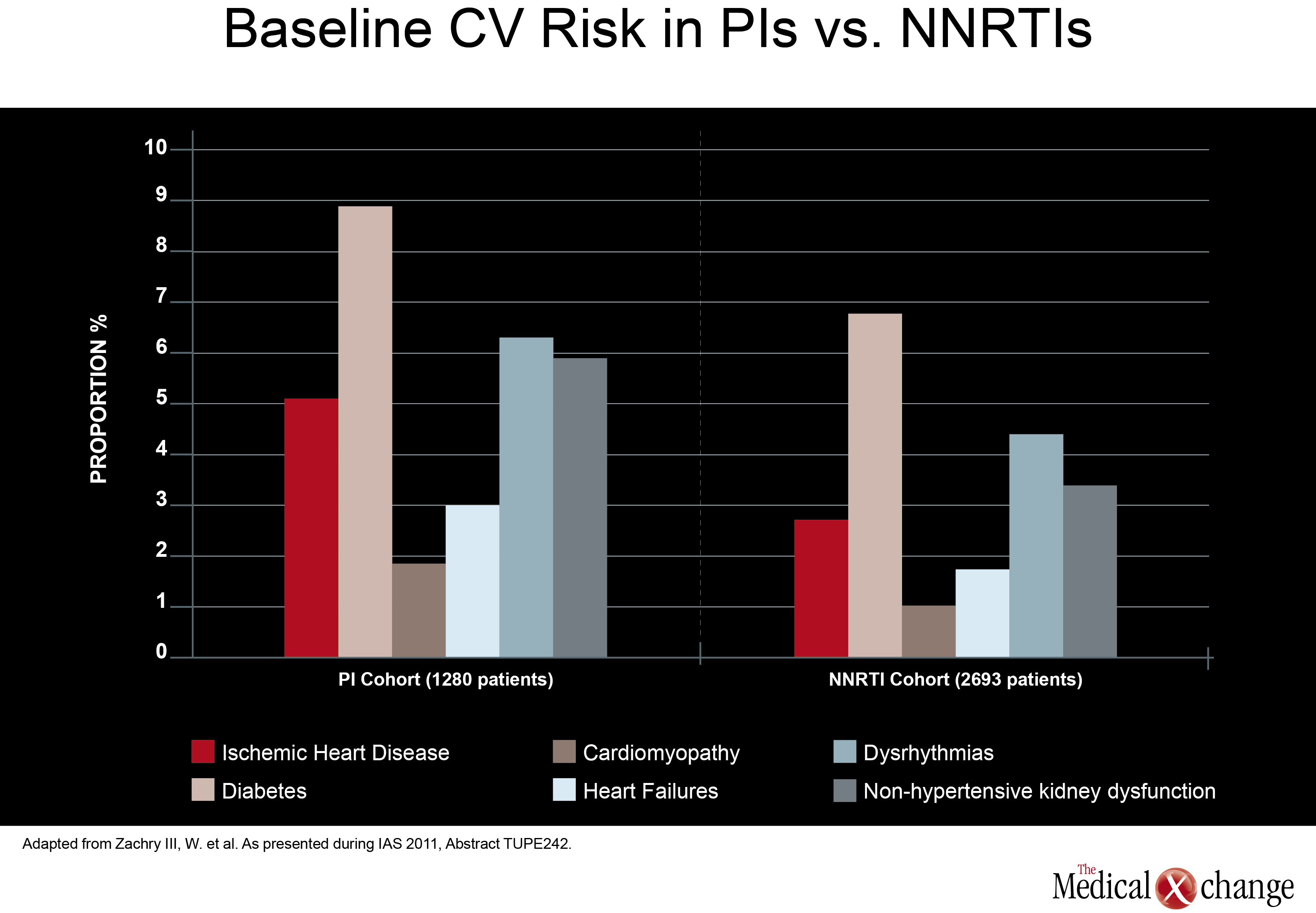
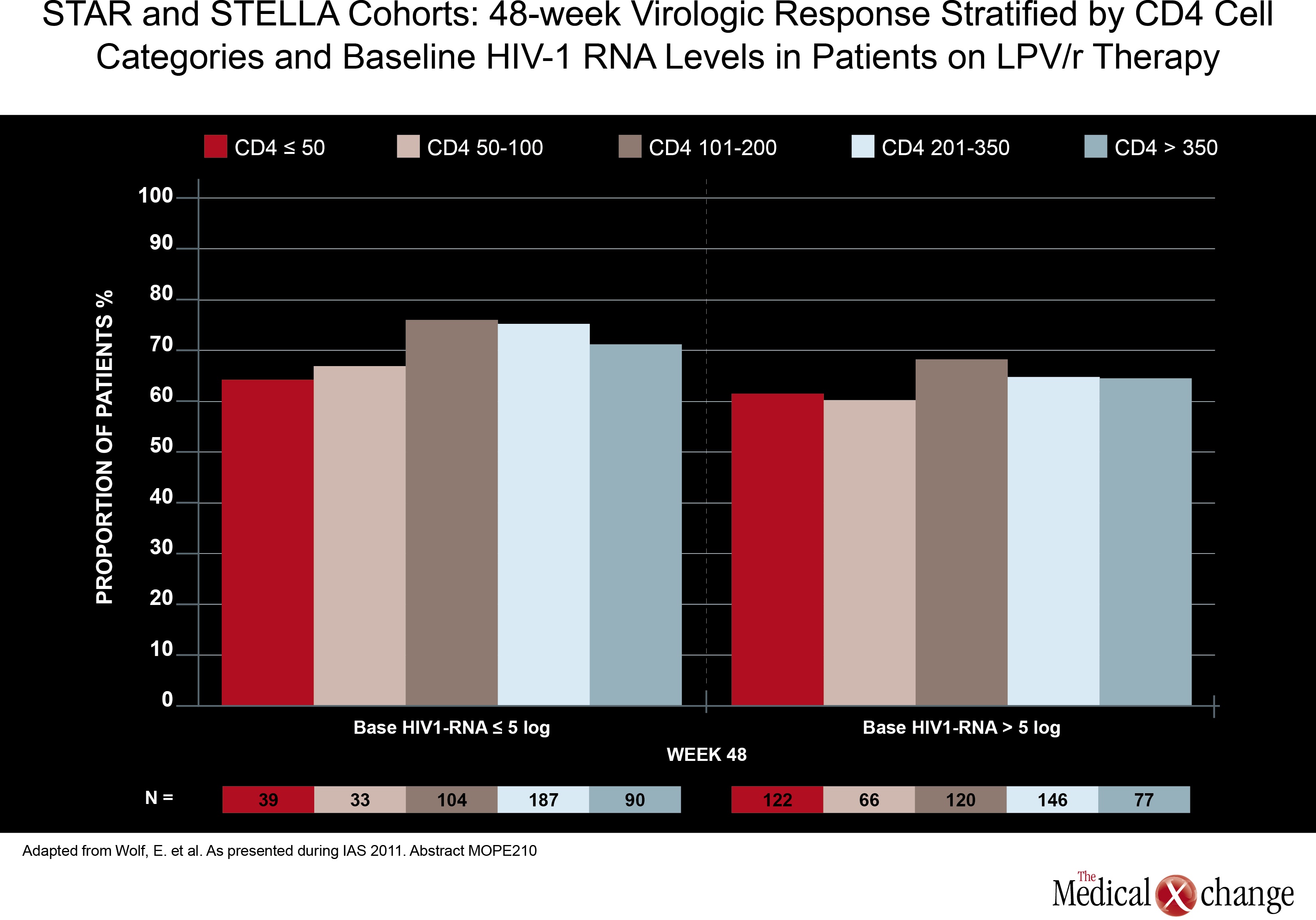
In direct comparisons of traditional PI-anchored three-drug regimens, there was little attention paid at the 2011 IAS meeting to relative effects on cardiovascular (CV) risk. Although renal impairment is a CV risk factor, it is well recognized that aging HIV patients should be closely monitored for all CV risk factors, regardless of regimen. However, one study, led by a team of investigators from Abbott Laboratories did find higher baseline CV risk in patients treated with a PI compared to those treated with a NNRTI. In data derived from a medical claims database, those in the PI cohort (1280 patients) had a greater baseline prevalence of ischemic heart disease (5.08% vs. 2.67%; P< 0.001), diabetes (8.83% vs. 6.76%; P=0.02), cardiomyopathy (1.80% vs. 0.97%; P=0.026), heart failure (2.97% vs. 1.67%; P=0.008), dysrhythmias (6.25% vs. 4.34%; P=0.01), and non-hypertensive kidney dysfunction (5.86% vs. 3.34%;P< 0.001) when compared to those in the NNRTI cohort (2693 patients) (Fig. 2). Also, more patients in the PI cohort vs. the NNRTI cohort had a history of HIV-related conditions (17.73% vs. 12.33%; P1 ARV prescriptions yielded similar results. Although the authors did not speculate on the reasons why patients prescribed PIs had increased baseline prevalence of CV risk factors, CV-related morbidity and HIV-related morbidity when compared with subjects receiving NNRTIs, these results may help future observational analyses better control for potential confounders when investigating antiretroviral-associated CV risk. Again, there was no significant study comparing potency in two traditional triple-drug regimens anchored with a PI, but one study did assess the potency of a ritonavir-boosted LPV (LPV/r)-based regimen in patients with low baseline CD4 counts. Although a previous study had suggested less treatment success when CD4 counts are low at treatment initiation, data from the German STAR and STELLA cohorts reached a different conclusion. In this study, led by Dr. Eva Wolf, MUC Research, Munich, Germany, no relationship was seen between 48-week treatment success (HIV suppression at <50 HIV RNA copies/mL) and baseline CD4 counts in 984 treatment-naive patients treated with a LPV/r-based regimen. The efficacy of LPV/r across baseline strata was seen both in univariate analysis and after adjusting for variables such as age, NRTI backbone, and viral load (Fig. 3). More data have been made available to challenge the once prominent belief that EFV-based regimens may not be appropriate in patients with high viral loads or low baseline CD4 cell counts. In an open-label study, 87 treatment-naive patients with a baseline CD4 count <100 cells/mm3 were randomized to EFV, LPV/r, or ATV/r plus TDF and emtricitabine (FTC). At 48 weeks, the proportion of patients with optimal HIV suppression (<50 HIV RNA copies/mL) was 79% for EFV, 62% for LPV/r. and 53% for ATV/r. The immune reconstitution expressed as cells/mm3 was greatest on LPV/r (226), but was not significantly different from the rates on EFV (199) and ATV/r (186). The authors of the study, led by Dr. José M. Miro, Hospital Clinic i Provincial, Barcelona, Spain, confirmed that EFV should not be considered less potent than PIs in highly immunocompromised patients.
Protease Inhibitors in Novel Approaches
One of the growing trends in PI application is NRTI-sparing regimens in which a PI is used as monotherapy or combined with such therapies as integrase inhibitors or the CCR5 antagonist maraviroc. In 144-week results from the MONET trial, which compared DRV/r monotherapy to DRV/r plus 2 NRTIs in patients with HIV RNA <50 copies/mL at baseline, monotherapy showed non-inferior efficacy in the switch-included analysis, but did not show non-inferior efficacy as compared to combination therapy (69.3% vs. 75.2% <50 copies/mL) when switch was counted as failure as defined by time to loss of virologic response (TLOVR) on an intention-to-treat (ITT) analysis in which switch=failure). Additionally, monotherapy performed far less well in those who had hepatitis C infection (HCV+) (43.5% vs. 73.3%) when the same type of analysis (TLOVR, switch=failure) was used. Although DRV/r monotherapy failed to show non-inferiority on the primary TLOVR switch=failure analysis, the authors of the study, led by Dr. José R. Arribas, Hospital la Paz, Madrid, Spain, indicated that boosted PI monotherapy may be an option in well-controlled patients when there is a good reason to avoid NRTIs. Selective application may also be appropriate.
The presence of HCV did not significantly increase failure rates in LPV/r patients randomized to discontinue NRTIs when compared to those who remained on triple therapy.
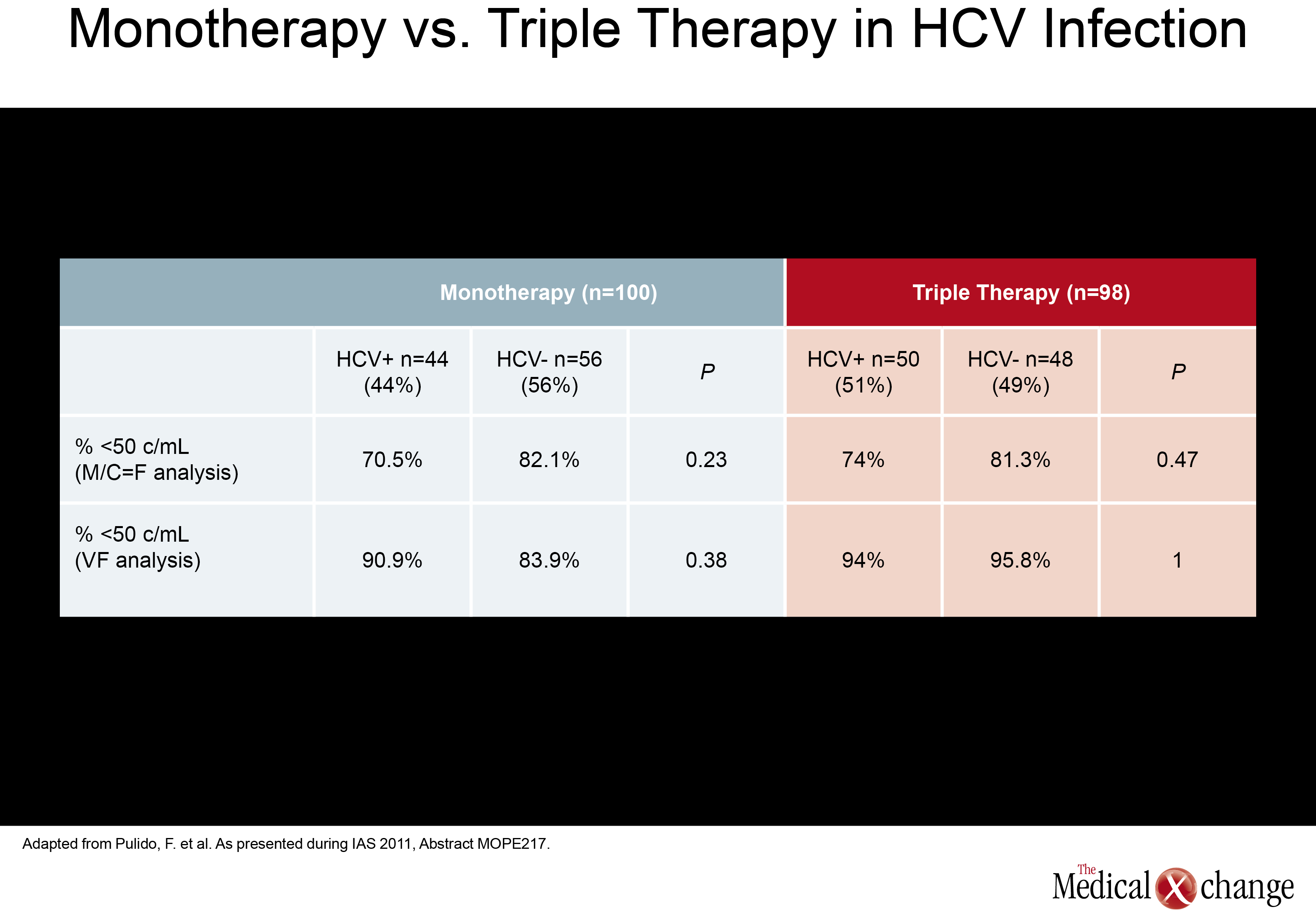
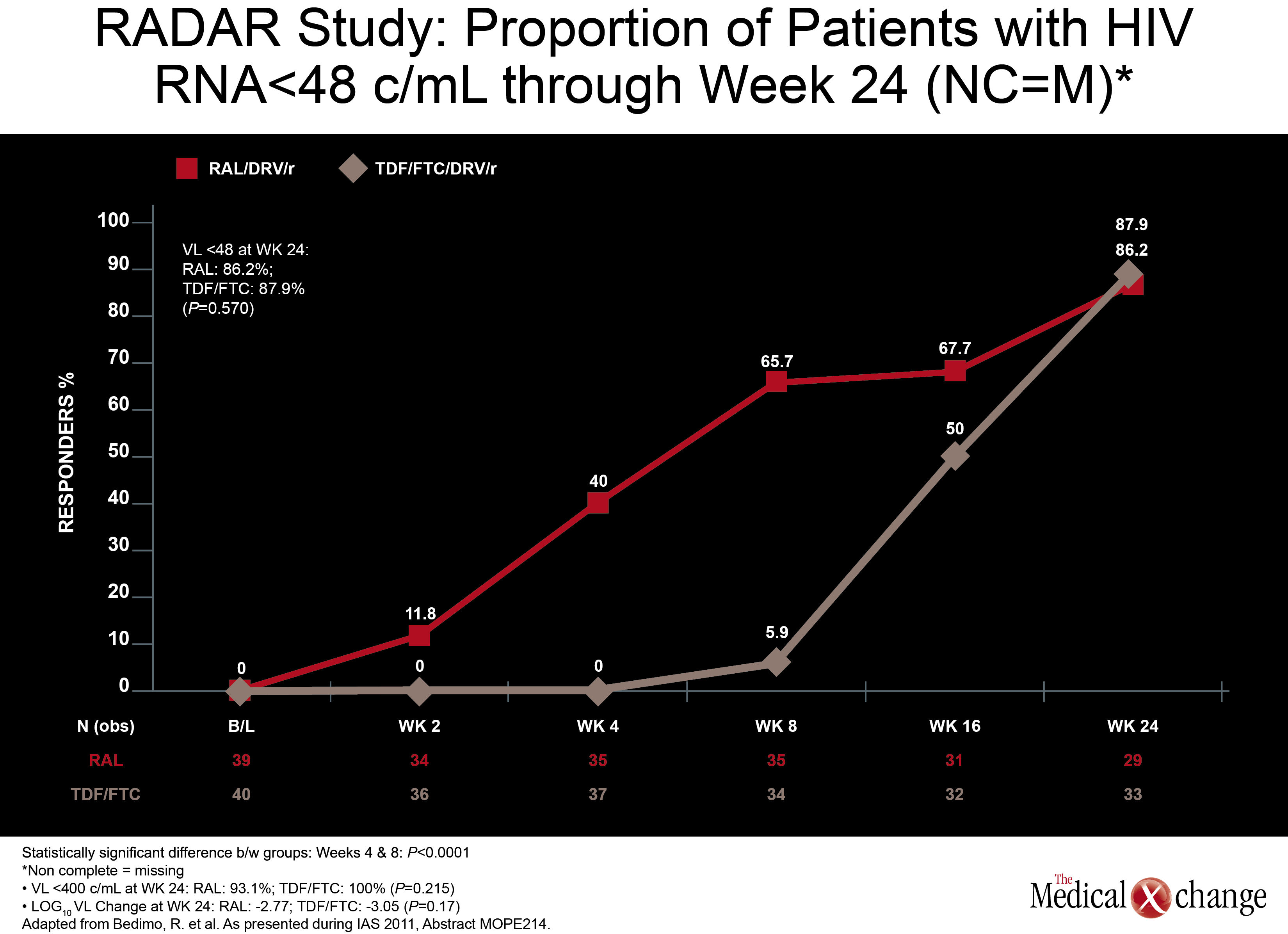
In a monotherapy trial with LPV/r, which is among PIs frequently used in NRTI-sparing regimens, HCV+ did not significantly increase failure rates. In this study, called OK04, patients receiving LPV/r plus two NRTIs were randomized to remain on their regimen or to discontinue the NRTIs and remain on LPV/r as monotherapy. While the proportions of patients with HIV RNA <50 copies/mL were higher on triple therapy in both HCV+ and HCV- groups (94% and 95.8%, respectively), they were not statistically different in the LPV/r monotherapy group (90.9% and 83.9%, respectively; P=0.38) in the ITT analysis (Table 2). In the per protocol analysis, differences were even smaller. The authors of the study, led by Dr. Federico Pulido, Hospital 12 de Octubre, Madrid, Spain, concluded that although a non-significant trend towards higher failure rates in the HCV+ population was seen in the ITT analysis, LPV/r monotherapy may be an option in selected HCV+ patients when it is well tolerated and patients remain adherent. (Fig. 4) Another NRTI-sparing study, called RADAR, looked at the combination of DRV/r plus the integrase inhibitor raltegravir (RGV) versus DRV/r plus TDF/FTC. Led by Dr. Roger Bedimo, VA North Texas Healthcare System, Dallas, Texas, the study team randomized 79 patients to one of the two regimens. Viral load reductions overall were far faster on the NRTI-free combination, with 65.7% vs. 5.9% reaching target suppression (100,000 copies/mL and below. These data are reassuring because the ACTG 5262 results reported at the 2011 Conference on Retroviruses and Opportunistic Infections (CROI) suggested suboptimal efficacy for this regimen in the presence of high viral loads when tested in a single-arm study. An appropriately powered double-blind study is needed to confirm that this is a reasonable alternative regimen in treatment-naive patients.
While nearly 20% of those on the arm that included TDF/FTC had at least a 5% decrease in total BMD, the proportion was under 5% in the RGV/LPV/r arm.
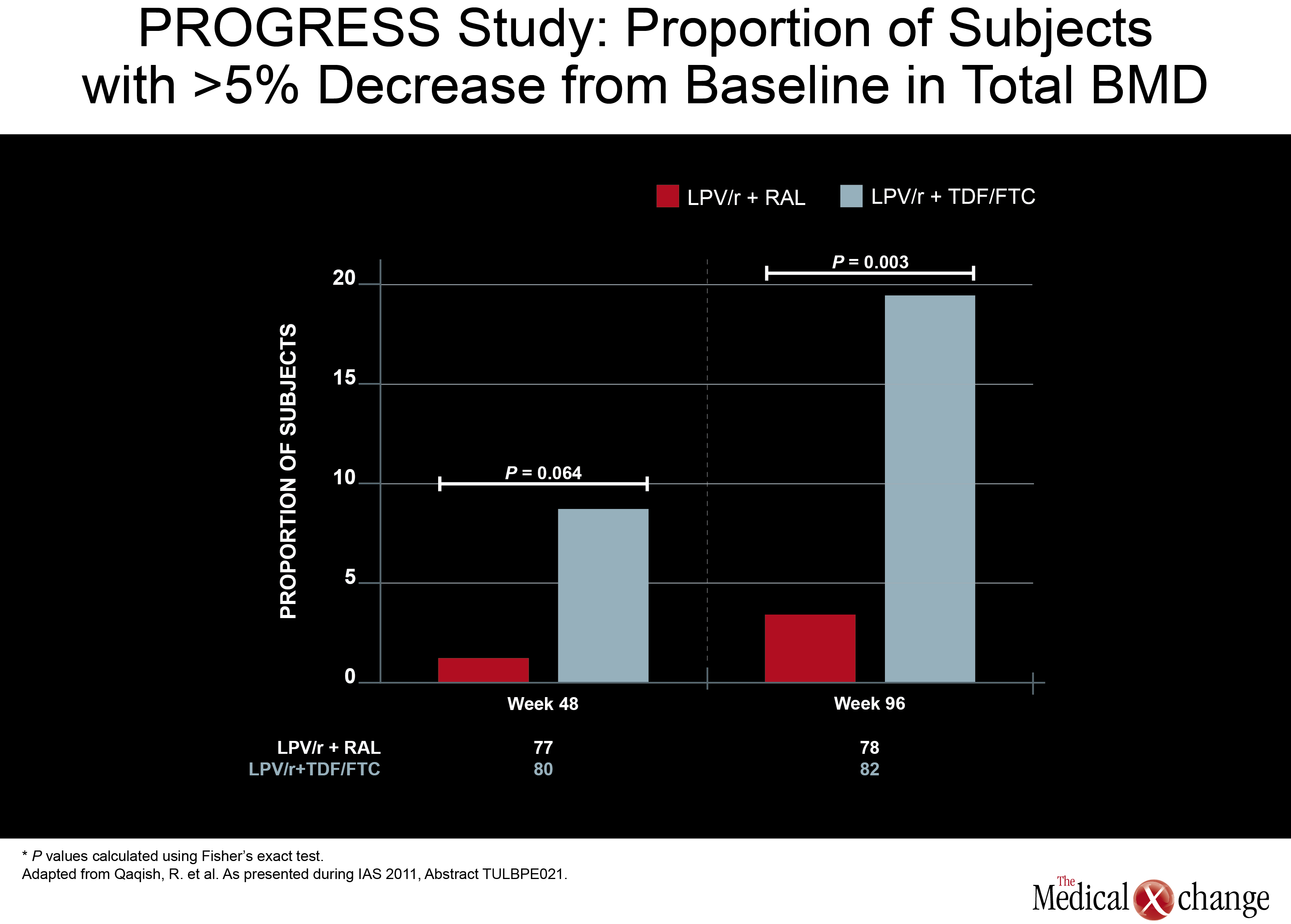
Another NRTI-sparing study, called PROGRESS, provided some insight of where this strategy may be most useful. In this study, 160 treatment-naive HIV patients were randomized to LPV/r plus RGV or to a traditional PI regimen of LPV/r plus TDF/FTC. HIV control at 96-weeks, like earlier timepoints, was similar, but new data presented by an investigating team led by Dr. Roula B. Qaqish found a difference in bone mineral density (BMD) loss. While nearly 20% of those on the arm that included TDF/FTC had at least a 5% decrease in total BMD, this level of bone loss was observed in less than 5% in the RGV/LPV/r arm. ART-naïve patients initiating LPV/r with raltegravir (RAL)had no significant loss of total body BMD and significantly smaller reduction in spine BMD compared to those who received LPV/r plus TDF/FTC. The minimal effect of RAL in combination with LPV/r on BMD warrants confirmation through further study (Fig. 5). In a phase 2b study, a NRTI-sparing regimen containing ATV/r plus the CCR5 antagonist maraviroc was found to produce an acceptable and sustained rate of viral suppression. In the multicenter study, 121 patients with documented CCR5- tropic infection were randomized to once-daily regimens with either ATV/r plus maraviroc or ATV/r plus TDF/FTC. The primary endpoint was viral load <50 copies/mL. At 48 weeks, this endpoint was achieved in 74.5% of those taking the NRTI-sparing regimen and 83.6% of those taking the regimen containing NRTIs. The proportion with a viral load <400 copies/mL was 89.8% and 86.9%, respectively. Both rates were similar to those reported at 24 weeks. The authors, led by Dr. Simon Portsmouth, a researcher with Pfizer, reported that creatinine clearance, although stable in the NRTI-sparing regimen, declined in those randomized to the regimen containing TDF/FTC. However, the rate of serious adverse events between groups was similar and more patients in the NRTI-sparing arm had treatment-related grade 3 or 4 adverse events (18 vs. 11). Still, due to the consistency of viral suppression over 48 weeks in the NRTI-sparing arm, the authors indicated that this approach may be viable when an NRTI-sparing approach is attractive.
Conclusion
As viral suppression can now be reliably achieved with a variety of effective regimens, the attention paid to the relative safety of antiretroviral therapy has been intensified by the aging of HIV patient populations. In data from the San Francisco registry reported at the 2011 IAS meeting, the proportion of patients over the age of 50 living with AIDS had climbed above 50% for the first time. The authors of that study, led by Dr. Susan Scheer, San Francisco Department of Public Health, California, predicted that the trend will continue and suggested that the growing population of older persons with HIV will place new challenges on medical care. PIs have a well established history of potency leading to long-term HIV suppression. They remain well poised to continue to be part of strategies to sustain HIV control. Clinical studies are on-going to evaluate how these drugs can slow or circumvent the risks associated with accelerated aging.
Protease Inhibitors after 15 Years: Report from the IAS
Rome – In 1996, a protease inhibitor (PI)-based antiretroviral combination led to the first opportunity for long-term survival for most patients living with human immunodeficiency virus (HIV). On the 15th anniversary of that breakthrough, PIs are no longer a mandatory component of an effective antiretroviral regimen, but they remain an important and reliable option in HIV control. As they are among the most potent antiretroviral agents, PIs remain a reasonable choice in a wide variety of clinical scenarios, including first-line treatment. Although some of the initially introduced PIs remain available, the common PIs in most formularies are ritonavir-boosted atazanavir (ATV/r), darunavir (DRV/r), and lopinavir (LPV/r). Relative to first generation PIs, these have few dosing restrictions, a low pill burden, and less relative risk of adverse events. Despite the broad experience with all of these agents, optimal use of PIs remains a dynamic exploration of relative strengths and weaknesses, particularly in the context of an aging HIV-infected population. This update assembles key studies on PIs from the 2011 IAS meeting.
Show review