Expert Review
Severe Asthma: Characterization for Individualized Therapy
Chapter 2: Severe Asthma Endotypes
Harold Kim, MD, FRCPC
Division of Clinical Immunology and Allergy, Western University and McMaster University,
London and Hamilton, Ontario
Precision medicine that addresses underlying pathophysiological mechanisms in patients with asthma is dependent on progress in defining endotypes. Unlike the descriptive phenotypes of asthma that have long been used to group patients by observable characteristics, the term endotypes recognizes distinct and potentially targetable pathophysiological mechanisms. In asthma, the identification of meaningful endotypes remains at an early stage, but there has been progress. Biomarkers predicting a greater likelihood of response to currently available targeted therapies provided an initial step toward individualized therapy. As an umbrella term for a complex and heterogeneous set of pathologic processes, asthma, particularly in its severe forms, is unlikely to be distilled into endotypes defined by single molecular mechanisms. Rather, as molecular, genetic, and epigenetic events are employed to distinguish endotypes, the goal will be to identify key processes that can be targeted for precision medicine in those with shared disease features.
Background
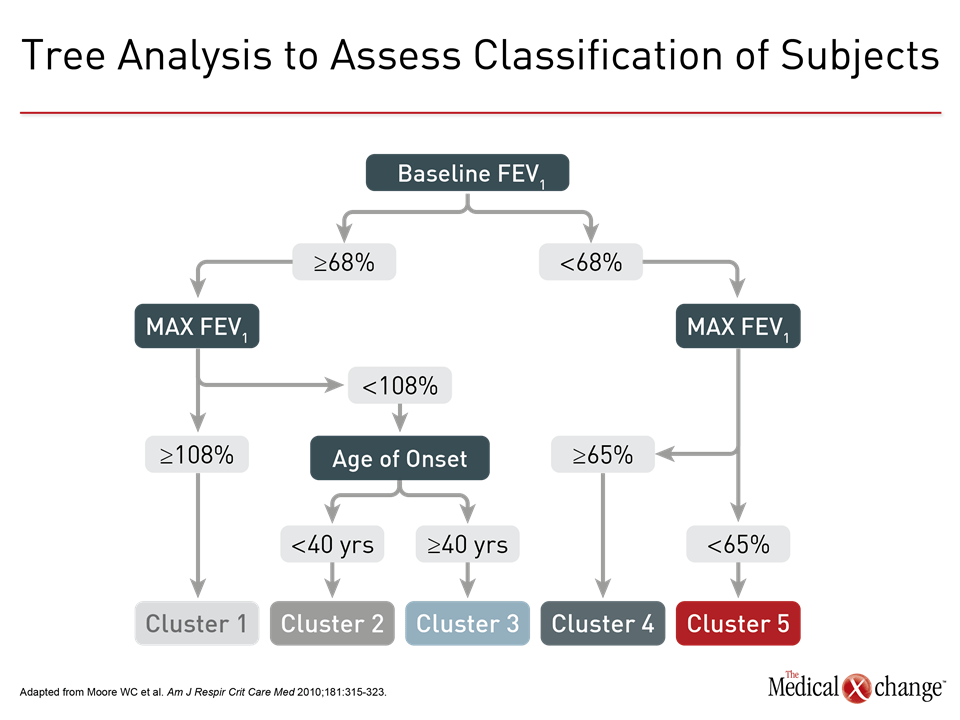
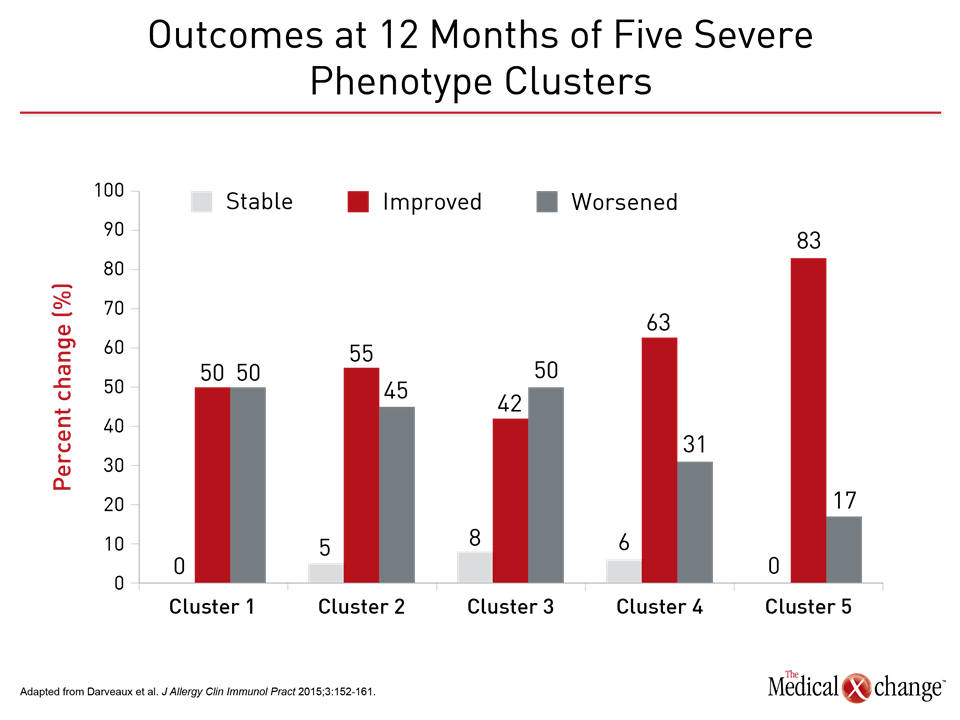
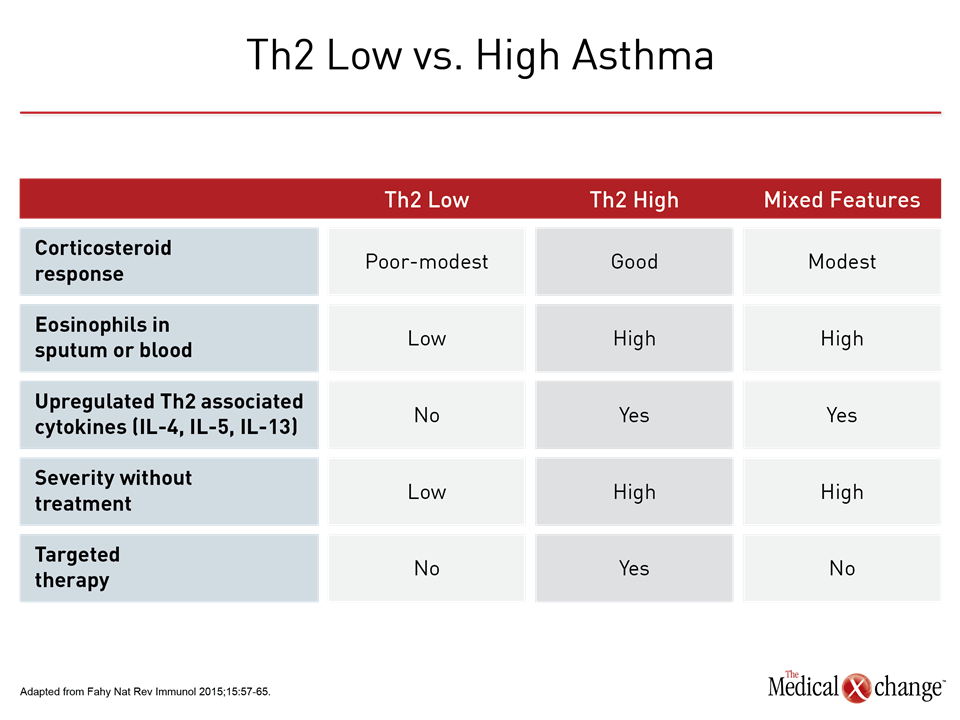
The effort to employ phenotypes as a strategy to address the heterogeneity of asthma has been underway for more than 50 years.1 As clinical features, such as age of onset, response to therapy, or symptom triggers, proved insufficient for meaningful clinical differentiation, particularly in severe disease, phenotyping has grown in complexity to accommodate multiple disease characteristics. The on-going Severe Asthma Research Program (SARP) is an example. In one early SARP analysis, several hundred clinical variables were distilled into 34 core variables and then evaluated through a statistical-based cluster analysis.2 Five distinct clusters were described based on clinical, physiologic, and inflammatory parameters (Fig. 1). These and other strategies to improve phenotyping have provided compelling evidence that asthma is a heterogeneous disease likely to involve distinct sets of pathophysiologic mechanisms. However, the specific phenotypes so far described involve overlapping characteristics. In the SARP clusters, the highly significant between-group differences in clinical features, including lung function, median age of asthma onset, and median body mass index, were not exclusionary but reflected relative differences, limiting their value for characterizing disease in individual patients. In a subsequent longitudinal evaluation that compared SARP clusters by clinical outcomes, there were no differences over a 12-month observation period 3 (Fig. 2). This included time to first exacerbation and asthma control according to the Asthma Control Questionnaire (ACQ). Phenotyping does correlate modestly with clinical outcome. In one example, five phenotypes were compared for patient-rated asthma control at 12 months.4 A frequent exacerbator phenotype was associated with poorer symptom control than an early-onset phenotype. Although the authors concluded that the findings suggest phenotypes may be useful for predicting outcome, their study did not evaluate whether modifications in therapy based on phenotypes could have improved outcomes or evaluate the value of phenotyping in the individual patient as opposed to the between-group differences that were observed. The authors acknowledged that validating studies are needed. In this study as well as the initial SARP analysis, phenotyping was performed solely on the basis of observable clinical characteristics. Phenotyping that includes biomarkers, sometimes referred to a molecular phenotypes,5 have the potential to identify more distinct asthma subtypes. Biomarkers may reflect underlying pathophysiologic events even if they do not necessarily reveal the degree to which these events drive disease. In fact, molecular phenotypes and endotypes are related and not always treated differently. Low and high T-helper cell type 2 (Th2) asthma is based on clinical characteristics and biomarkers reflecting immune system activity (Table 1). Some recent studies have characterized these terms as phenotypes, while others have described them as endotypes.6,7 Inconsistency in the use of the term endotype is understandable. The Th2 low and high subtypes, proposed nearly 20 years ago,8 were based on both clinical characteristics and inflammatory biomarkers at time when it was less clear that asthma was likely to result from more than one pathophysiologic pathway. Endotype has been a term more recently introduced, recognizing that there are distinct mechanisms of disease likely to require distinct treatment strategies.9 The description and investigation of Th2 high and low asthma has provided much of the framework for pursuing meaningful endotypes even if these terms are not adequately specific for defining treatment targets.
Biomarkers for Endotyping
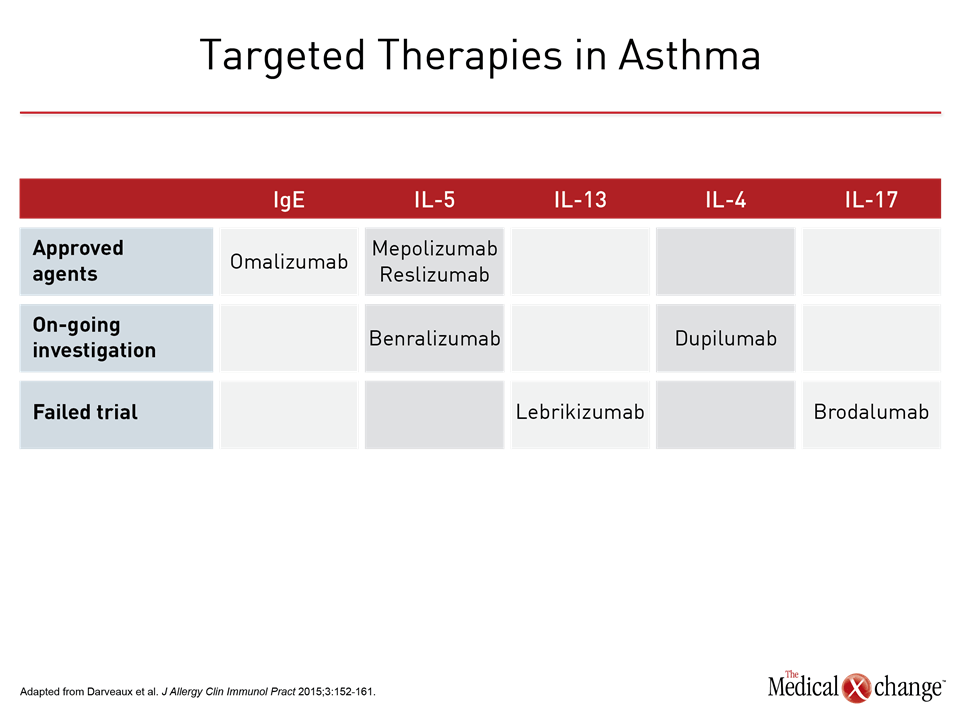
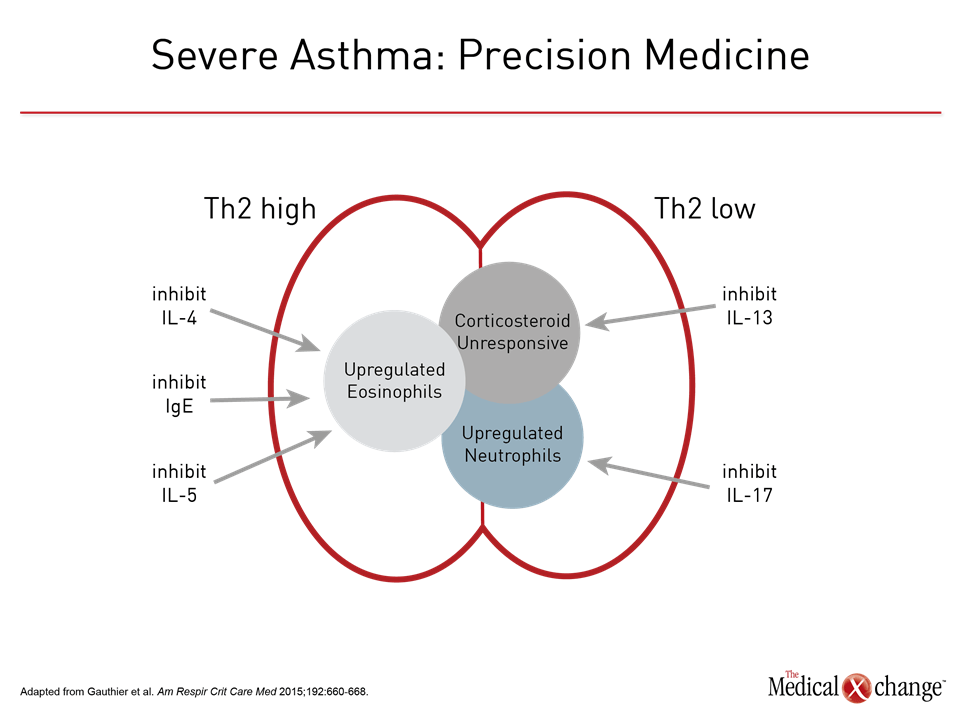
The observation that some but not all patients with asthma develop airway eosinophilia was published in 1958.10 Subsequent studies associated eosinophilia with good response to corticosteroids and low levels of eosinophils in the airway to a poor response.11,12 Subsequent studies demonstrated that the presence or absence of eosinophils were related to related events in the inflammatory pathway, such as relative expression of the cytokines interleukin-4 (IL-4) and IL-5.13 These observations eventually led to a now widely accepted distinction between Th2 high and low asthma.14 This stratification is important, because it suggests there are fundamental differences in the immune system activation that drive airway inflammation and bronchial spasm in patients with clinical asthma. The classical view of asthma as a Th2-mediated disease is based on the premise that some exposure or event, such as a viral respiratory tract infection, activates this subset of T helper cells to release an antibody response of the IgE class along with such cytokines as IL-4, IL-5, and IL-13, thereby triggering eosinophil activation and mobilization. Unlike Th1 mediated immunity, which is associated with release of interferon gamma, IL-2, and tumor necrosis-factor in order to induce cell-mediated immunity,15 Th2 responses are more closely aligned with allergic responses and have led to the theory that asthma, particularly asthma with a childhood onset, is the result of some trigger of inappropriate Th2 activation.16 Although this Th2 eosinophilic predominant asthma, described as Th2 high disease, has long been considered the classical form of asthma, only about 50% of cases fit this description.17 Patients without Th2 high features, or Th2 low asthma, have been identified by a variety of means, starting with a low blood or sputum eosinophil count, which have been defined variably. In one analysis of non-eosinophilic asthma in the general population, the cut-off was <2% eosinophils in the sputum,18 but higher counts have been used to identify patients with severe eosinophilic asthma who are candidates biologic therapy for which the presence of eosinophilia is a requirement.19 Other approaches have included poor response to corticosteroids, low expression of Th2-associated cytokines, such as IL-5, and a gene expression profile linked to non-Th2 high asthma.20 The weakness of defining Th2 low and high asthma as endotypes is that they appear to lack specificity. Biologics targeted at IgE and the IL-5 pathway, which are upregulated in Th2 high asthma, are effective, but response correlates imperfectly with the presence of the target, such as elevated IgE in the case of omalizumab or eosinophils in the case of the IL-5 pathway inhibitors mepolizumab and reslizumab.21-23 Omalizumab has shown efficacy in reducing allergic responses without regard to specific allergen,24 but the presence of elevated IgE is not specifically required in current labeling for the treatment of asthma. Labeling for the IL-5 pathway inhibitors do specify the presence of eosinophilia, but the presence of eosinophilia does not guarantee a response.25 In one analysis, sputum eosinophil counts did not predict a treatment response to mepolizumab.26 Lebrikizumab, which targets IL-13, failed to provide consistent benefits in a phase 2 asthma trials even though it is also associated with down regulation of eosinophil activation.27 Dupilumab, an inhibitor of IL-4 and IL-13 signaling, has shown clinical activity in severe asthma independent of eosinophil count28 (Table 2). The evidence supporting Th2 low asthma as an endotype is even less compelling. Although it has been hypothesized that Th2 low inflammation is caused by dysregulation in the innate immune response, resulting in an inflammation predominated by upregulation of neutrophils,29 the precise sequence of events and the potential for targetable events in this sequence remains poorly understood. In animal and human studies, upregulation of IL-17, which is known to induce cytokines and chemokines important to the activation and mobilization of neutrophils, has been observed in non-eosinophilic asthma,30 but a clinical study with the anti-IL-17 antibody brodalumab did not demonstrate a treatment effect.31 In opposition to a phenotype, endotype requires pathophysiologic mechanisms, and these have yet to be well defined by simple Th2 high and low categories. Other phenotypes, such as late onset asthma, obesity-associated asthma, or exercise-induced asthma, may be defined by endotypes unrelated or indirectly related to Th2 status. Exercise-induced asthma, for example, is linked to upregulation of inflammatory mediators more closely associated with edema and bronchoconstriction, such as leukotrienes and prostaglandins.32 In these clinical classifications of asthma, like the Th2 low and high phenotypes, clinically useful endotypes may require definitions dependent on multiple characteristics. The complexity of these characteristics may increase with severity. In children with severe asthma, for example, both eosinophils and neutrophils can be elevated, blurring a difference based on characterization by T helper cell inflammation.33
Clinical Summary
The progress toward endotyping is based on the increasing detail with which the pathophysiology of different phenotypes of asthma is understood. This progress is expected to unravel the mechanisms by which pathologic processes lead to disease expression. Although a detailed understanding of the pathophysiology of asthma may eventually lead to strategies for preventing initial triggers, no immediate goal is more important than reversing drivers of inflammation in severe asthma (Fig. 3). Severe asthma, generally defined as poor disease control despite high doses of corticosteroids, only occurs in 10% or fewer of patients, but it is responsible for a high proportion of urgent care visits,34 imposing a measurable toll in asthma-related deaths in Canada as elsewhere.35 The introduction of targeted therapies has been an important step toward the definition of endotypes and precision medicine. In particular, the IL-5 pathway inhibitors have been associated with large and clinically significant reductions in exacerbations resulting in emergency department visits or hospitalizations.36-38 Elevated eosinophil levels have been a predictor of benefit, thereby establishing the relevance of the target. It is reasonable to predict that suppression of the IL-5 pathway of eosinophil activation will be one of many steps toward suppressing very specific inflammatory mediators linked to this and the additional endotypes likely to emerge from efforts to trace asthma pathophysiology. There has been enormous progress in identifying the components of the inflammatory cascade. The next steps involve transforming asthma phenotypes into asthma endotypes on the basis of an understanding of which components in the inflammatory cascade drive these asthma subtypes. In turn, these may provide targets of treatment specific to that endotype, expanding the opportunities for precision medicine for a disease or set of diseases that have proven to be remarkably complex.
Conclusion
As designation for symptoms that develop from a heterogeneous set of pathophysiologic processes, the term asthma may be as non-specific as the term cancer for defining a disease entity. The importance of recognizing differences in the underlying pathology is reflected in current ERS/ATS guidelines for severe asthma, which repeatedly endorse phenotyping as a strategy to individualize therapy.39 Endotyping relies of a precise understanding of the specific pathophysiologic pathways of asthma. Endotyping remains at an early stage of development, but it is likely to become a tool for substantially improving the treatment of asthma. The contribution of endotyping is particularly promising for its potential to lead to new treatment options for severe disease, which, by definition, has been poorly responsive to traditional therapies.
References
- Gauthier M, Ray A, Wenzel SE. Evolving Concepts of Asthma. Am J Respir Crit Care Med 2015;192:660-8.
- Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010;181:315-23.
- Bourdin A, Molinari N, Vachier I, et al. Prognostic value of cluster analysis of severe asthma phenotypes. J Allergy Clin Immunol 2014;134:1043-50.
- Khusial RJ, Sont JK, Loijmans RJB, et al. Longitudinal outcomes of different asthma phenotypes in primary care, an observational study. NPJ Prim Care Respir Med 2017;27:55.
- Papi A, Saetta M, Fabbri L. Severe asthma: phenotyping to endotyping or vice versa? Eur Respir J 2017;49.
- Kuo CS, Pavlidis S, Loza M, et al. T-helper cell type 2 (Th2) and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in U-BIOPRED. Eur Respir J 2017;49.
- Stokes JR, Casale TB. Characterization of asthma endotypes: implications for therapy. Ann Allergy Asthma Immunol 2016;117:121-5.
- Wenzel SE, Schwartz LB, Langmack EL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 1999;160:1001-8.
- Lotvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol 2011;127:355-60.
- Brown HM. Treatment of chronic asthma with prednisolone; significance of eosinophils in the sputum. Lancet 1958;2:1245-7.
- Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet 2002;360:1715-21.
- Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet 1999;353:2213-4.
- Leung DY, Martin RJ, Szefler SJ, et al. Dysregulation of interleukin 4, interleukin 5, and interferon gamma gene expression in steroid-resistant asthma. J Exp Med 1995;181:33-40.
- Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009;180:388-95.
- Romagnani S. T-cell subsets (Th1 versus Th2). Ann Allergy Asthma Immunol 2000;85:9-18; quiz , 21.
- Barnes PJ. Th2 cytokines and asthma: an introduction. Respir Res 2001;2:64-5.
- Pearce N, Douwes J, Beasley R. Is allergen exposure the major primary cause of asthma? Thorax 2000;55:424-31.
- Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax 2002;57:643-8.
- Choy MS, Dixit D, Bridgeman MB. Mepolizumab (Nucala) For Severe Eosinophilic Asthma. P T 2016;41:619-22.
- Chung KF. Personalised medicine in asthma: time for action: Number 1 in the Series “Personalised medicine in respiratory diseases” Edited by Renaud Louis and Nicolas Roche. Eur Respir Rev 2017;26.
- Chapman KR, Cartier A, Hebert J, McIvor RA, Schellenberg RR. The role of omalizumab in the treatment of severe allergic asthma. Can Respir J 2006;13 Suppl B:1B-9B.
- Deeks ED. Mepolizumab: A Review in Eosinophilic Asthma. BioDrugs 2016;30:361-70.
- Mukherjee M, Sehmi R, Nair P. Anti-IL5 therapy for asthma and beyond. World Allergy Organ J 2014;7:32.
- Casale TB, Bernstein IL, Busse WW, et al. Use of an anti-IgE humanized monoclonal antibody in ragweed-induced allergic rhinitis. J Allergy Clin Immunol 1997;100:110-21.
- Darveaux J, Busse WW. Biologics in asthma–the next step toward personalized treatment. J Allergy Clin Immunol Pract 2015;3:152-60; quiz 61.
- Katz LE, Gleich GJ, Hartley BF, Yancey SW, Ortega HG. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc 2014;11:531-6.
- Hanania NA, Korenblat P, Chapman KR, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med 2016;4:781-96.
- Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016;388:31-44.
- Ciepiela O, Ostafin M, Demkow U. Neutrophils in asthma–a review. Respir Physiol Neurobiol 2015;209:13-6.
- Agache I, Akdis CA. Endotypes of allergic diseases and asthma: An important step in building blocks for the future of precision medicine. Allergol Int 2016;65:243-52.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med 2013;188:1294-302.
- Boulet LP, O’Byrne PM. Asthma and exercise-induced bronchoconstriction in athletes. N Engl J Med 2015;372:641-8.
- Fitzpatrick AM, Higgins M, Holguin F, et al. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol 2010;125:851-7 e18.
- Ray A, Raundhal M, Oriss TB, Ray P, Wenzel SE. Current concepts of severe asthma. J Clin Invest 2016;126:2394-403.
- To T, Simatovic J, Zhu J, et al. Asthma deaths in a large provincial health system. A 10-year population-based study. Ann Am Thorac Soc 2014;11:1210-7.
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014;371:1198-207.
- Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015;3:355-66.
- FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016;388:2128-41.
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343-73.
Chapter 2: Severe Asthma Endotypes
Precision medicine that addresses underlying pathophysiological mechanisms in patients with asthma is dependent on progress in defining endotypes. Unlike the descriptive phenotypes of asthma that have long been used to group patients by observable characteristics, the term endotypes recognizes distinct and potentially targetable pathophysiological mechanisms. In asthma, the identification of meaningful endotypes remains at an early stage, but there has been progress. Biomarkers predicting a greater likelihood of response to currently available targeted therapies provided an initial step toward individualized therapy. As an umbrella term for a complex and heterogeneous set of pathologic processes, asthma, particularly in its severe forms, is unlikely to be distilled into endotypes defined by single molecular mechanisms. Rather, as molecular, genetic, and epigenetic events are employed to distinguish endotypes, the goal will be to identify key processes that can be targeted for precision medicine in those with shared disease features.
Show review