Expert Review
Pursuing the Next Step in Reduction of Residual CV Risk
Chapter 2: The Development and Promise of PCSK9 Inhibitors
Nabil G. Seidah, OC, OQ, PhD, FRSC
Director, Laboratory of Biochemical Neuroendocrinology Clinical Research Institute of Montreal, Canada
Research Chair in Proteolysis Montreal, QC
Low-density lipoprotein cholesterol (LDL-C) concentrations in the blood are to a large degree controlled by the activity of LDL-C cell surface receptors (LDLr). When bound and removed from the circulation by these receptors, LDL-C is no longer available as a substrate for atherosclerosis. Increasing the activity of LDLr is the principle of the lipid-lowering proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. PCSK9 is a protein that enhances LDLr degradation. By inhibiting PCSK9, LDLr activity is preserved, increasing the amount of LDL-C removed from the circulation. Monoclonal antibodies to PCSK9 in clinical trials have produced sustained reductions in LDL-C exceeding those typically achieved with HMG-CoA reductase inhibitors (statins). The trajectory of PCSK9 discovery and clinical development of targeted inhibitors has been an exceptional demonstration of the ability of molecular biology to rapidly develop novel therapies for human pathology.
Background and History
The first characterization of the ninth member of the proprotein convertase family, proprotein convertase subtilisin/kexin type 9 (PCSK9), was published in 2003.1 PCSK9, which was initially labeled neural apoptosis-regulated convertase 1 (NARC-1), was isolated before its biological function was understood, but a rapid series of genetic discoveries of PCSK9 mutants established that this enzyme played an important role in cholesterol metabolism.2,3 Subsequent studies more specifically demonstrated that PCSK9 binds to and then degrades the LDL-C receptor.4,5 Less than 10 years after its initial characterization, a phase 1 clinical trial with a monoclonal antibody to PCSK9 showed significant LDL-C lowering activity in human subjects.6
Less than 10 years after its initial characterization, a phase 1 clinical trial with a monoclonal antibody to PCSK9 showed significant LDL-C lowering activity in human subjects.
PCSK9 is expressed by a limited number of cells that include hepatocytes, kidney mesenchymal, and colon epithelia.1 Its role in LDL-C metabolism was pursued after PCSK9 gene mutations were identified in two families with hypercholesterolemia.2 These gain-of-function (GOF) mutations suggested that greater PCSK9 activity results in an increase in circulating LDL-C levels. Loss-of-function (LOF) PCSK9 mutations were later associated with the opposite effect, joining an accumulating body of evidence that this enzyme is an important mediator of circulating LDL-C.3 The potential clinical relevance of these findings was further emphasized when the presence GOF or LOF PCSK9 mutations were associated with higher and lower rates, respectively, of CV events relative to those without these mutations.3,7 In one study, for example, the presence of a LOF PCSK9 nonsense mutation found in approximately 3% of Caucasians was associated with a 15% reduction in LDL-C and a 47% reduction in coronary heart disease (CHD).3 Although somewhat less common in African-Americans, the same mutation was associated with even greater protection from CHD in this population (Fig. 1). LDLr binds to the LDL-C particle and, through endocytosis, eliminates the particle from the circulation.8 Typically, PCSK9 binds to LDLr and is internalized along with the LDL-C particle (Fig. 2).9,10 In the cell, PCSK9 induces a change in LDLr conformation that subjects the receptor to lysosomal degradation, which eliminates its physiological function. In the absence of PCSK9, LDLr is returned to the cell surface where it can again bind to LDL-C, sustaining its activity. The direct inverse correlation between the activity of this escort protein and circulating levels of LDL-C makes it an attractive target for cholesterol-lowering treatment strategies. PCSK9 is primarily synthesized in the liver, but it may have biological functions other than regulation of LDL-C. In experimental studies, for example, PCSK9 activity has been implicated in triglyceride metabolism and regulation of cholesterol balance in adipocytes and enterocytes.11,12 So far, there is no clear signal in human studies that loss of PCSK9 function imposes detrimental effects on cholesterol metabolism or other biological activities. Although such detrimental effects cannot yet be ruled out, otherwise healthy individuals with no detectable PCSK9 due to multiple LOF PCSK9 mutations have been identified in two reports.13,14 In both, complete absence of PCSK9 was associated with a LDL-C level of approximately 0.4 mmol/L.13
Clinical Trials Programs
Multiple clinical trials programs with monoclonal antibodies (mAbs) to PCSK9 have validated PCSK9 as a target for achieving reductions in LDL-C. Although other strategies for inhibiting the activity of PCSK9 have been or are being pursued, including antisense oligonucleotides, small interfering RNA (siRNA), small peptide inhibitors, and adnectins,10,15,16 these remain in early phase or preclinical studies. The large trial programs with mAbs, including phase 3 clinical trials, have confirmed large and sustained reductions in LDL-C with PCSK9 inhibition.
The large trial programs with mAbs, including phase 3 clinical trials, have confirmed large and sustained reductions in LDL-C with PCSK9 inhibition.
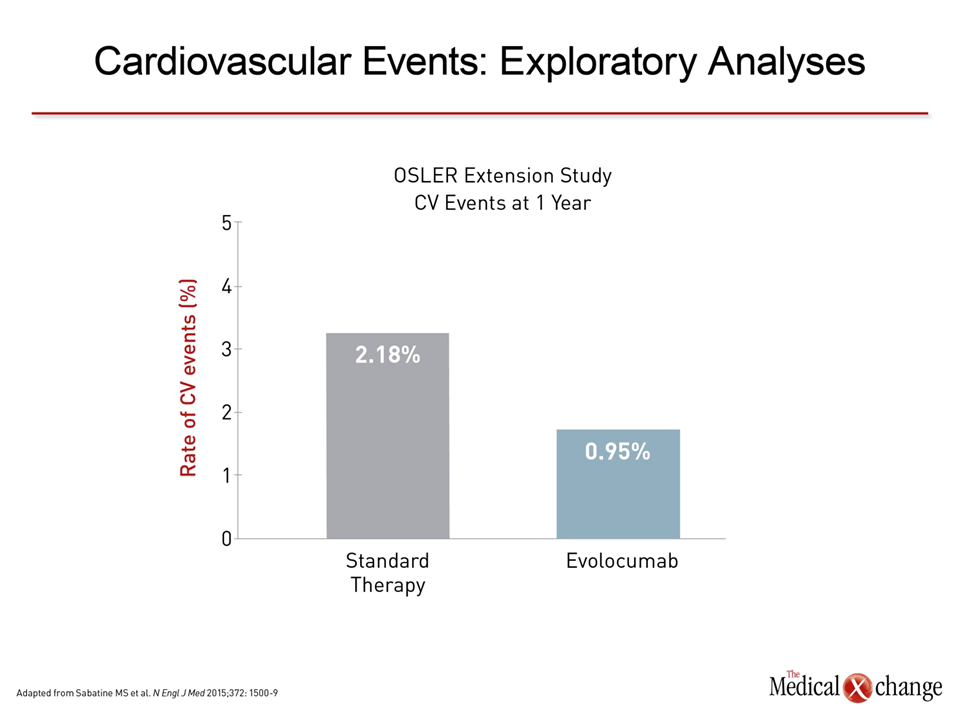
In one of the first series of clinical experiences published, reductions in LDL-C ranged in a dose-dependent manner from 28.1% to 65.4% after a single intravenous dose of the PCSK9 inhibitor alirocumab.6In this series, data was summarized from three phase 1 clinical trials conducted in both healthy volunteers and individuals with familial hypercholesterolemia already on atorvastatin. In one study of multiple doses, sustained reductions in LDL-C were observed over the study period when alirocumab was administered on days 1, 29, and 43. In familial hypercholesterolemia patients taking atorvastatin, the effects of the PCSK9 inhibitor were largely additive. On the basis of this and other clinical studies, a phase 3 development program called ODYSSEY was initiated. One of the largest studies completed to date, ODYSSEY Long-Term, randomized 2,341 patients at 320 participating sites in 27 countries.17 Relative to placebo, 150 mg of alirocumab administered every 2 weeks was associated with a 62.1% reduction in LDL-C. The treatment effect was consistent over the course of the 78-week trial (Fig. 3). The most common adverse events more frequently observed on alirocumab than placebo were injection-site reactions (5.9% vs. 4.2%) and myalgia (5.4% vs. 2.9%). Other adverse events were infrequent, although both neurocognitive (1.2% vs. 0.5%) and ophthalmologic events (2.9% vs. 1.9%) were numerically higher on alirocumab. A post-hoc analysis associated alirocumab with a reduction of major adverse CV events (1.7% vs. 3.3%; P=0.02) consistent with its lipid-lowering activity (Fig. 4a). Other already completed phase 3 trials from the ODYSSEY program include ODYSSEY COMBO I,18 ODYSSEY COMBO II,18 and ODYSSEY OPTIONS 1.19 All placebo-controlled studies conducted in patients at high risk for CV events, the COMBO studies associated alirocumab with large reductions in LDL-C among patients taking maximally-tolerated statins and the OPTIONS study associated alirocumab with greater reductions in LDL-C than other lipid-lowering strategies when each was added to a high-intensity statin (atorvastatin or rosuvastatin). In the ODYSSEY ALTERNATIVE trial, which has so far only been presented in abstract form,20 alirocumab was associated with large and sustained reductions in LDL-C among patients intolerant to statins (Fig. 5). The ODYSSEY OUTCOMES trial, which is designed specifically to evaluate the ability of alirocumab to prevent CV events, is ongoing. In that multinational trial, approximately 18,000 high-risk patients who have had an acute coronary syndrome within the previous 52 weeks have been randomized to alirocumab or placebo on top of standard therapies for dyslipidemia. The primary composite endpoint includes CHD death, non-fatal myocardial infarction (MI), fatal and non-fatal ischemic stroke, and hospitalization for unstable angina. Results are expected in early 2018. Randomized studies have also now been conducted with the mAbs evolocumab and bococizumab. Of these, the largest body of evidence is available for evolocumab, for which combined data were published from open-label extensions of phase 2 (OSLER-1) and phase 3 (OSLER-2) studies.21 In the combined data from these extension studies with 4,465 patients, evolocumab in doses of 140 mg every two weeks or 420 mg once per month were associated with large and sustained reductions in LDL-C with a low rate of adverse events. Although the extension studies were not randomized, numerically higher rates of arthralgia (4.6% vs. 3.2%), headache (3.6% vs. 2.1%) and fatigue (2.8% vs. 1.0%) were observed on evolocumab relative to standard therapy. Rates of serious adverse events were low for both, but neurocognitive events were again numerically higher among individuals taking evolocumab (0.9% vs. 0.3%). As in ODYSSEY LONG-TERM, a non-randomized evaluation of CV events in the OSLER extension studies suggested an advantage for the PCSK9 inhibitor over usual therapy (Fig. 4b). In a placebo-controlled, dose-ranging study of bococizumab, reductions in LDL-C appeared to be on the same order of magnitude as that observed previously with alirocumab and evolocumab.22 Adverse events in this 24-week study were observed in low frequency and at rates that were generally comparable to placebo. Based on the study, phase 3 bococizumab clinical trials are planned with every 2-week subcutaneous dosing. Findings similar to those from the individual studies were produced by a meta-analysis combining data from 12,200 patients participating in 25 randomized controlled trials with alirocumab or evolocumab.23Reductions in LDL-C have ranged from approximately 50% to 60%. Although it is essential to reserve judgment about long-term safety until large sets of clinical data are accumulated over several years, no significant safety concerns were identified. Overall, the evidence to date creates a strong likelihood that PCSK9 inhibitors will provide the next major step forward in reducing CV events through lipid lowering, particularly in high-risk individuals.
Patients Who Will Benefit Most From PCSK9 Inhibitors
Statins, which are effective and well tolerated, permitted the landmark trials that have made reductions in LDL-C an essential step in CV risk reduction, but many individuals, particularly those at highest risk of CV events, cannot reach the guideline-based targets established by those trials. In Canada, one survey found 40% of high-risk patients, most of whom on statin therapy, were below their guideline-recommended goal.24 These data suggest a substantial unmet need for additional effective and well-tolerated lipid-lowering therapies. The data accumulated so far suggest that PCSK9 inhibitors may provide a major contribution to CV risk reduction simply by increasing the proportion of patients able to reach current treatment goals. The reasons for failing to reach goals on statins include absolute and relative intolerance, particularly at the highest doses of statins, and insufficient potency when baseline levels of LDL-C are particularly elevated. PCSK9 inhibitors also have potential to improve LDL-C control in some forms of familial hypercholesterolemia. There are already supportive data for all of these clinical applications. For those not adequately compliant to once-daily statin therapy taken orally, a subcutaneous injection of a PCSK9 inhibitor every 2 to 4 weeks may be a viable alternative. PCSK9 inhibitors also offer an opportunity to explore the value of reducing LDL-C below the levels routinely attainable in high-risk patients on statins alone. In a recent trial evaluating the non-statin ezetimibe on top of statin, patients in the experimental arm achieved a median LDL-C of 1.4 mmol/L, which was associated with an incremental reduction in CV events relative to a LDL-C of 1.8 mmol/L achieved in the arm receiving high-intensity statins alone.25 Due to the limitations of currently available lipid-lowering therapies, the optimal level of LDL-C has yet to be established. PCSK9 inhibitors may play a role in redefining the maximum CV risk reductions achievable through LDL-C control.
Summary
PCSK9 inhibitors have the potential to address an important need in the prevention of CV events. Relative to statins in randomized trials, PCSK9 inhibitors have produced greater reductions in LDL-C and have been at least as well tolerated. Whether used as alternatives to statins or in combination with statins, PCSK9 inhibitors appear likely to substantially increase the proportion of patients at risk of CV events who achieve maximum protection through LDL-C control. The potential for these agents to redefine optimal LDL-C levels in patients at high risk of CV disease is likely to be a focus in future clinical trials.
References
1. Seidah NG, Benjannet S, Wickham L, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A 2003;100:928-33. 2. Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003;34:154-6. 3. Cohen JC, Boerwinkle E, Mosley TH, Jr., Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264-72. 4. Tavori H, Fan D, Blakemore JL, et al. Serum proprotein convertase subtilisin/kexin type 9 and cell surface low-density lipoprotein receptor: evidence for a reciprocal regulation. Circulation 2013;127:2403-13. 5. Maxwell KN, Breslow JL. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci U S A 2004;101:7100-5. 6. Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med 2012;366:1108-18. 7. Tibolla G, Norata GD, Artali R, Meneghetti F, Catapano AL. Proprotein convertase subtilisin/kexin type 9 (PCSK9): from structure-function relation to therapeutic inhibition. Nutr Metab Cardiovasc Dis 2011;21:835-43. 8. Seidah NG. PCSK9 as a therapeutic target of dyslipidemia. Expert Opin Ther Targets 2009;13:19-28. 9. Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade. J Lipid Res 2012;53:2515-24. 10. Seidah NG, Awan Z, Chretien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res 2014;114:1022-36. 11. Roubtsova A, Munkonda MN, Awan Z, et al. Circulating proprotein convertase subtilisin/kexin 9 (PCSK9) regulates VLDLR protein and triglyceride accumulation in visceral adipose tissue. Arteriosclerosis, thrombosis, and vascular biology 2011;31:785-91. 12. Levy E, Ben Djoudi Ouadda A, Spahis S, et al. PCSK9 plays a significant role in cholesterol homeostasis and lipid transport in intestinal epithelial cells. Atherosclerosis 2013;227:297-306. 13. Zhao Z, Tuakli-Wosornu Y, Lagace TA, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. American journal of human genetics 2006;79:514-23. 14. Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis 2007;193:445-8. 15. Hooper AJ, Burnett JR. Anti-PCSK9 therapies for the treatment of hypercholesterolemia. Expert opinion on biological therapy 2013;13:429-35. 16. Frank-Kamenetsky M, Grefhorst A, Anderson NN, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci U S A 2008;105:11915-20. 17. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489-99. 18. Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. Am Heart J 2015;169:906-15 e13. 19. Bays H, Gaudet D, Weiss R, et al. Alirocumab as Add-on To Atorvastatin Versus Other Lipid Treatment Strategies: ODYSSEY OPTIONS I Randomized Trial. The Journal of clinical endocrinology and metabolism 2015:jc20151520. 20. Moriarty PM, Jacobson TA, Bruckert E, Thompson PD. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 monoclonal antibody alirocumab versus ezetemibe in patients with statin intolerance as defned by a placebo run-in and statin rechallenge arm. American Heart Association. Chicago: LBCT02; 2014. 21. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500-9. 22. Ballantyne CM, Neutel J, Cropp A, et al. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo-controlled, dose-ranging study in statin-treated subjects with hypercholesterolemia. Am J Cardiol 2015;115:1212-21. 23. Zhang XL, Zhu QQ, Zhu L, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC medicine 2015;13:123. 24. Goodman SG, Langer A, Bastien NR, et al. Prevalence of dyslipidemia in statin-treated patients in Canada: results of the DYSlipidemia International Study (DYSIS). Can J Cardiol 2010;26:e330-5. 25. Cannon CP, Cariou B, Blom D, et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. European heart journal 2015;36:1186-94.
Chapter 2: The Development and Promise of PCSK9 Inhibitors
Low-density lipoprotein cholesterol (LDL-C) concentrations in the blood are to a large degree controlled by the activity of LDL-C cell surface receptors (LDLr). When bound and removed from the circulation by these receptors, LDL-C is no longer available as a substrate for atherosclerosis. Increasing the activity of LDLr is the principle of the lipid-lowering proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. PCSK9 is a protein that enhances LDLr degradation. By inhibiting PCSK9, LDLr activity is preserved, increasing the amount of LDL-C removed from the circulation. Monoclonal antibodies to PCSK9 in clinical trials have produced sustained reductions in LDL-C exceeding those typically achieved with HMG-CoA reductase inhibitors (statins). The trajectory of PCSK9 discovery and clinical development of targeted inhibitors has been an exceptional demonstration of the ability of molecular biology to rapidly develop novel therapies for human pathology.
Show review