Expert Review
Solving Osteoarthritis Pain Control: Current Status of Hyaluronic Acid Knee Injections
Chapter 3: Hyaluronic Acid in Clinical Practice: Managing Symptoms in Knee Osteoarthritis
Michael Clarfield, MD, CCFP
Director, Sports Medicine Specialists, Toronto, Ontario
The therapeutic goals in the management of osteoarthritis (OA) of the knee are to control pain and minimize functional limitations. Treatment is individualized with attention to the immediate objective of pain control while minimizing the risks of the prolonged therapies that may be required to control this chronic condition. Of pharmacologic therapies available for the treatment of knee OA, injection of hyaluronic acid (HA) offers a favorable balance of efficacy and safety. Unlike other conservative treatment options with efficacy against OA pain, such as non-steroidal anti-inflammatory drugs (NSAIDs), localized delivery of HA is associated with a low risk of local or systemic adverse effects. New generation HA therapies, relative to HA formulations introduced in Europe and the United States in the late 1990s, have several relative advantages, including a long duration of effect after a single injection and in some cases a more rapid onset of pain control. Characteristic differences among new generation agents may be further relevant to individualized care in selected patients.
Background
Osteoarthritis (OA) is often misunderstood as a condition of older individuals. Although deterioration of the cartilage leading to OA can be age-related, there are a broad number of etiologies that initiate the progressive events that characterize this condition. Currently, the median age at diagnosis of OA is 55, (1) but knee injuries that damage cartilage, ligaments or menisci, upsetting the balance of interrelated joint structures that drives progressive OA, can occur at any age. (2) The altered biomechanics of a limb can produce uneven stress on the joint causing early degeneration. Not all knee injuries lead to OA, which is multifactorial and may include a genetic predisposition, (3) but a variety of stresses to the knee, including injury, markedly increase risk. (4) Clinical knee OA may evolve a decade or more after a single index injury, (5) but early trauma or stress to the knee means that OA rates begin climbing in the third decade of life with case reports common at earlier ages. (6)
With evidence that knee OA can develop after traumatic knee injury, substantial attention has been paid to the risk posed by athletic injuries for early onset OA. In a review of the relationship between surgically-repaired anterior cruciate ligament (ACL) tears and knee OA, for example, it was concluded that isolated injuries pose a low risk, but risk of OA climbs markedly among those with both an ACL tear and another structural knee injury. (7)
However, traumatic injury is not the only insult that leads to initial degradation of articular cartilage. Obesity, which is increasingly common in many countries, including Canada, (8) is also associated with increased risk of early onset OA. (9) The biomechanical stress imposed by obesity on the knee is substantiated by reports of articular cartilage damage in obese teenagers. (10) In addition to stress, obesity, a pro-inflammatory condition, may further exacerbate joint equilibrium by upregulating factors contributing to OA pathology. (11)
The treatment of knee OA in relatively young patients underscores the challenges of balancing efficacy and safety over prolonged periods, which is an issue that may be equally relevant to an older population. Long-term treatment strategies are necessary, because OA is typically incurable and progressive. The speed at which OA progresses varies substantially, (12) but damage in most patients eventually extends to other joint tissues, including the synovial membrane, muscles, ligaments, and bone. (13) Non-pharmacological therapies, such as weight loss in the obese or muscle strengthening in those with traumatic injuries, are an important adjunct for slowing or halting OA progression. (14,15) While the immediate objective is pain relief and restoring or improving joint function, a combination of pharmacologic and non-pharmacologic interventions should be targeted at achieving stable, non-progressive disease (Fig. 1).
Knee Osteoarthritis: Clinical Goals
Relative to clinical findings alone, radiographic studies are useful for improving the accuracy of the diagnosis of knee OA, (16) but there is a poor correlation between symptoms and radiographic severity as judged with the widely used Kellgren and Lawrence Classification system. (17) From the more practical clinical perspective in patients with established knee OA, reproducible methods developed for evaluating the clinical burden of knee OA may be useful. The Western Ontario and McMaster Universities Arthritis Index (WOMAC), which measures pain, stiffness, function, and global symptomatology, is accurate, (18, 19) but many clinicians may be comfortable guiding or modifying treatment plans on the basis of patient report alone.
Due to the variability in the manifestations of OA, clinical goals may differ. Although knee OA is the leading cause of disability in Canada and the United States, (20, 21) the definitions of disabling pain and joint stiffness are subjective. Regaining function may be a more important goal for an athlete with early onset OA and stiffness than an elderly individual whose dominant complaint is pain. However, the risk of adverse events from chronic treatment regimens is an issue for both. In relatively young patients, attention must be paid to disease control over decades. In the elderly, treatment selection requires sensitivity to the greater relative susceptibility of patients in this age group to acute adverse events.
OA at the present time cannot be cured, but there is interest in management that will slow or halt the biomechanical and biochemical events triggered by cartilage loss. These events include inflammation, joint destabilization, changes in joint alignment, and bone remodeling. (22) The only proven strategies to achieve this goal are non-pharmacologic interventions, such as weight loss in the obese, that reduce injury to the articular cartilage. Novel treatments on the horizon, such as cell-based strategies to regenerate cartilage, (23) are driven by the goal of improving joint integrity to slow OA progression.
HA Injection in Control of Osteoarthritis
Injection of hyaluronic acid (HA), also called viscosupplementation, has been available in Canada for the treatment of knee OA for more than 10 years. Although only one option on a long list of pharmacologic therapies ranging from acetaminophen to opioids, it has been identified by the American Academy of Orthopaedic Surgeons (AAOS) as helpful for individuals with knee OA who have not responded to non-pharmacologic conservative measures or other basic treatments, such as acetaminophen. (24) The AAOS guidelines, which recommend HA for mild to moderate knee OA, note that HA is a naturally-occurring lubricant that is generally found in diminished concentration among patients with OA. Consistent with previously-published studies, (25, 26) the benefit from HA, as described by the AAOS, is derived from facilitating movement of knee joint components while improving shock absorption characteristics.
A review of 76 published studies with HA in the treatment of OA, of which 36 were against such active comparators as intra-articular corticosteroid injections, non-steroidal anti-inflammatory drugs (NSAIDs), and exercise, concluded that HA is effective with benefit on pain, function, and patient global assessment. (27) The authors further determined from this analysis that HA has a more prolonged clinical benefit than intra-articular corticosteroids, is as at least effective as most other pharmacologic therapies, and in general is associated with a low risk of systemic effects. However, they cautioned that there appears to be time-dependent and efficacy variability across products.
This variability is consistent with incremental advances in HA formulations. All of the initial formulations delivered HA in relatively low concentrations requiring multiple injections. Initial products were also of avian derivation. Subsequent HA formulations have been modified to increase duration of activity, reduce risk of adverse events, and to recreate a closer match to the biochemical activity of naturally-occurring HA, which plays a more active role in joint homeostasis than originally presumed (Fig. 2). (28) Although the contribution of HA to the viscoelastic properties of synovial fluid were an initial impetus to develop exogenous products, (29) there is a large and growing body of clinical and experimental evidence that HA may also ameliorate knee OA pain and function by modifying pain perception, (30) upregulating of inflammatory mediators, (31) and preventing remodeling of extracellular matrix. (32)
The optimal composition of HA has not been defined, but there is evidence that high molecular weight HA, which more closely approximates the endogenous form, provides greater bioactivity in OA patients than lower molecular weight formulations. (33) Recently, high molecular weight was found to provide greater joint lubrication in an experimental model. (34) The new generation HA products have been formulated therefore with higher molecular weights than the initial products.
More recent products have also been more likely to be derived from non-avian sources, avoiding the potential risks from allergies to poultry and egg proteins. Finally, the most recent products are employing higher concentrations, with the potential to increase the bioactivity of treatment, accelerate symptom control, and increase the duration of response. Of the most commonly used products, for example, Monovisc is injected in a dose of 80 mg whereas Synvisc-One is injected in a dose of 48 mg (Fig. 3).
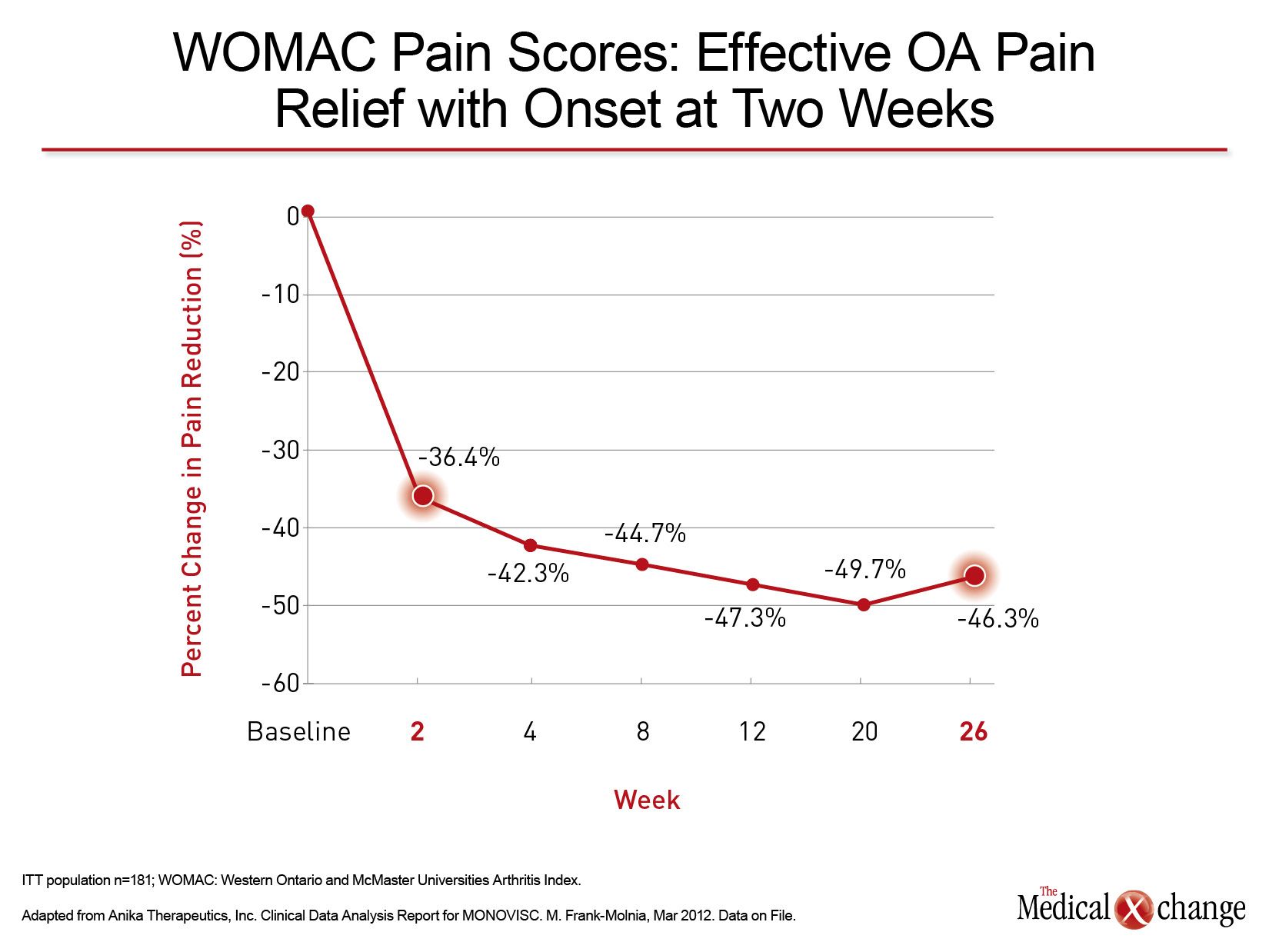
There are no large, randomized, double-blind studies comparing available HA formulations, but the relative effects of single agent, placebo-controlled trials are consistent with the anticipated advantages of high molecular weight, high concentration agents. In the registration trials with Monovisc, for example, there was a 36.4% improvement in pain scores at the week 2 visit, (35) which has not been reported for other agents. WOMAC pain scores continued to decline over a subsequent 18-week period with scores still suppressed below the 2-week decline at the end of 26 weeks (Fig. 4). The relatively rapid pain relief has important implications for the patient’s ability to appreciate the efficacy of HA and its effect on quality of life.
HA treatment is attractive for symptom control in mild to moderate disease when extended treatment encourages use of a well-tolerated therapy with a low risk of systemic effects. With current high molecular weight and high concentration formulations, single injections may provide efficacy for periods of up to six months, circumventing the inconvenience and discomfort of frequent retreatments. In addition to HA therapy it is recommended that patients also receive adjuvant strategies. These include muscle-strengthening exercises in the context of physical therapy and knee bracing. While long-term benefit from braces has not been demonstrated in a controlled trial, this approach has been shown to reduce joint loading and improve gait symmetry.(36) In some patients, particularly those unable or unwilling to complete physical therapy, patients should also be counselled to modify activity to reduce stress on the damaged joint.
Conclusion
OA of the knee is a challenging condition for which the immediate goals of therapy must be considered in the context of risk of progression and long-term management. HA is one of many options, but its attributes include a level of symptom control at least comparable to NSAIDs and intra-articular steroid injections. Replacing an endogenous compound important to joint kinetics, exogenous HA also poses a low risk for systemic side effects. Newer non-avian formulations with a high molecular weight and high concentration may better reproduce the activities of HA in the joint relative to earlier generation products. When combined with non-pharmacologic interventions, HA may be a valuable tool in the effort to achieve sustained relief of pain, improve function, and reduce risk of disease progression.
CASE 1
35-Year-Old Male
Figure 5 (Fig. 5). An otherwise fit accountant with a history of increasing knee pain provides a history of joint stiffness that is limiting his ability to pursue recreational skiing. He has self-medicated with ibuprofen to control symptoms but reports frequent episodes of dyspepsia. A diagnosis of OA is made on the basis of classic clinical signs.
- Although patient is initially referred for strengthening exercises, persistent pain brings the patient back for additional symptom control.
- High molecular weight, high-concentration HA is injected with instructions to avoid high-intensity exercise for 48 hours and to monitor swelling.
- In a telephone follow-up 2 weeks after injection, patient reports symptom improvement but is still supplementing treatment with ibuprofen.
- Four weeks after therapy, patient removes himself from supplemental analgesics and intensifies strengthening regimen.
- Patient is instructed to return in 6 months, or earlier if symptoms recur.
CASE 2
60-Year-Old Female
Figure 6 (Fig. 6). Recently retired after 30 years of a demanding work schedule, a former real estate saleswoman’s adoption of a sedentary lifestyle has led to a body mass index (BMI) increase from 32 to 37 in just two years. She has type 2 diabetes and hypertension. Her many allergies include nuts, eggs, and tomatoes. Her prior history includes a gastrointestinal bleed. Upon radiological examination, the patient meets Kellgren and Lawrence criteria for grade 2 OA.
- Relatively contraindicated for a COX-2 inhibitor because of cardiovascular risk factors, the patient has self-medicated with opioid analgesics obtained for a prior foot injury and complains of poor daytime concentration.
- Trial of a high molecular weight, high-concentration, non-avian HA is initiated.
- Patient is instructed to take acetaminophen for pain control.
- In follow-up at two weeks, patient reports insufficient pain control and requests opioid. She accedes to recommendation to wait an additional two weeks, but remains non-compliant to weight loss recommendation.
- Pain control at three weeks is adequate but radiograph at 6 months shows substantial joint deterioration to Kellgren and Lawrence grade 3.
- Surgical options are considered.
References
1. Niinimaki TT, Eskelinen A, Ohtonen P, Junnila M, Leppilahti J. Incidence of osteotomies around the knee for the treatment of knee osteoarthritis: a 22-year population-based study. International orthopaedics 2012;36:1399-402.
2. Wilder FV, Hall BJ, Barrett JP, Jr., Lemrow NB. History of acute knee injury and osteoarthritis of the knee: a prospective epidemiological assessment. The Clearwater Osteoarthritis Study. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2002;10:611-6.
3. Spector TD, MacGregor AJ. Risk factors for osteoarthritis: genetics. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2004;12 Suppl A:S39-44.
4. Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. Journal of orthopaedic trauma 2006;20:739-44.
5. Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 1995;3:261-7.
6. Gelber AC, Hochberg MC, Mead LA, Wang NY , Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Annals of internal medicine 2000;133:321-8.
7. Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. The American journal of sports medicine 2009;37:1434-43.
8. Canada. Adult obesity prevalence in Canada and the United States. http://www.statcan.gc.ca/pub/82-625-x/2011001/article/11411-eng. htm: Statistics Canada; 2013.
9. Sutton PM, Holloway ES. The young osteoarthritic knee: dilemmas in management. BMC medicine 2013;11:14.
10. Widhalm HK, Seemann R, Hamboeck M, et al. Osteoarthritis in morbidly obese children and adolescents, an age-matched controlled study. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA 2014.
11. Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Current opinion in rheumatology 2010;22:533-7.
12. Wolfe F, Lane NE. The longterm outcome of osteoarthritis: rates and predictors of joint space narrowing in symptomatic patients with knee osteoarthritis. The Journal of rheumatology 2002;29:139-46.
13. Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis – an untreatable disease? Nature reviews Drug discovery 2005;4:331-44.
14. Christensen R, Astrup A, Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2005;13:20-7.
15. Roddy E, Zhang W, Doherty M. Aerobic walking or strengthening exercise for osteoarthritis of the knee? A systematic review. Annals of the rheumatic diseases 2005;64:544-8.
16. Roemer FW, Eckstein F, Hayashi D, Guermazi A. The role of imaging in osteoarthritis. Best practice & research Clinical rheumatology 2014;28:31-60.
17. Neogi T, Felson D, Niu J, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. Bmj 2009;339:b2844.
18. McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis and rheumatism 2001;45:453-61.
19. Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Annals of the rheumatic diseases 2005;64:29-33.
20. Life with Arthritis in Canada. Public Health Agency of Canada; 2010.
21. Centers for Disease C, Prevention. Prevalence and most common causes of disability among adults–United States, 2005. MMWR Morbidity and mortality weekly report 2009;58:421-6.
22. Bruyere O, Collette JH , Ethgen O, et al. Biochemical markers of bone and cartilage remodeling in prediction of longterm progression of knee osteoarthritis. The Journal of rheumatology 2003;30:1043-50.
23. Musumeci G, Castrogiovanni P, Leonardi R, et al. New perspectives for articular cartilage repair treatment through tissue engineering: A contemporary review. World journal of orthopedics 2014;5:80-8.
24. AAOS. Viscosupplementation treatment for arthritis. http://orthoinfoaaosorg/topiccfm?topic=a002172014.
25. Brzusek D, Petron D. Treating knee osteoarthritis with intraarticular hyaluronans. Current medical research and opinion 2008;24:3307-22.
26. Miller LE, Block JE. US-Approved Intra-Articular Hyaluronic Acid Injections are Safe and Effective in Patients with Knee Osteoarthritis: Systematic Review and Meta-Analysis of Randomized, Saline-Controlled Trials. Clinical medicine insights Arthritis and musculoskeletal disorders 2013;6:57-63.
27. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. The Cochrane database of systematic reviews 2006:CD005321.
28. Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis research & therapy 2003;5:54-67.
29. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. The Cochrane database of systematic reviews 2005:CD005321.
30. Pozo MA, Balazs EA, Belmonte C. Reduction of sensory responses to passive movements of inflamed knee joints by hylan, a hyaluronan derivative. Experimental brain research 1997;116:3-9.
31. Yasui T, Akatsuka M, Tobetto K, Hayaishi M, Ando T. The effect of hyaluronan on interleukin-1 alpha-induced prostaglandin E2 production in human osteoarthritic synovial cells. Agents and actions 1992;37:155-6.
32. Kawasaki K, Ochi M, Uchio Y, Adachi N, Matsusaki M. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. Journal of cellular physiology 1999;179:142-8.
33. Wang CT, Lin YT, Chiang BL, Lin YH , Hou SM. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2006;14:1237-47.
34. Elmorsy S, Funakoshi T, Sasazawa F, Todoh M, Tadano S, Iwasaki N. Chondroprotective effects of high-molecular-weight cross-linked hyaluronic acid in a rabbit knee osteoarthritis model. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2014;22:121-7.
35. Anika Therapeutics, Inc. Clinical Data Analysis Report for MONOVISC. M. Frank-Molnia, Mar 2012. Data on File. 36. Briem K, Ramsey DK. The role of bracing. Sports Med Arthrosc 2013;21:11-17.
Chapter 3: Hyaluronic Acid in Clinical Practice: Managing Symptoms in Knee Osteoarthritis
The therapeutic goals in the management of osteoarthritis (OA) of the knee are to control pain and minimize functional limitations. Treatment is individualized with attention to the immediate objective of pain control while minimizing the risks of the prolonged therapies that may be required to control this chronic condition. Of pharmacologic therapies available for the treatment of knee OA, injection of hyaluronic acid (HA) offers a favorable balance of efficacy and safety. Unlike other conservative treatment options with efficacy against OA pain, such as non-steroidal anti-inflammatory drugs (NSAIDs), localized delivery of HA is associated with a low risk of local or systemic adverse effects. New generation HA therapies, relative to HA formulations introduced in Europe and the United States in the late 1990s, have several relative advantages, including a long duration of effect after a single injection and in some cases a more rapid onset of pain control. Characteristic differences among new generation agents may be further relevant to individualized care in selected patients.
Show review