Expert Review
Solving Osteoarthritis Pain Control: Current Status of Hyaluronic Acid Knee Injections
Chapter 1: Osteoarthritis of the Knee: Addressing a Growing Epidemic
Pankaj Dhawan, MD, FRCSC
Physical Medicine and Rehabilitation, Vancouver, British Columbia
Osteoarthritis (OA) of the knee is a common orthopedic complaint, affecting about one in six adult Canadians.(1) A chronic and progressive inflammatory condition, OA is painful, restricts activity, and impairs quality of life. Due to its chronicity, it is essential to consider the long-term safety and tolerability of therapeutic options in the context of extended symptom control. Strategies to slow the pace of joint deterioration are being pursued, but the current focus of intervention is on symptom control. Traditionally, such simple analgesics as acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) have been the first-line option, but their efficacy is limited and there are safety concerns with chronic use. For localized joint injections, hyaluronic acid (HA), which is a component of normal joint physiology, has safety advantages over corticosteroids, particularly when repeated treatments are anticipated. The durability of the effect of hyaluronic acid is also an advantage relative to other options. The success of any therapy is likely to be increased with non-pharmacologic support, particularly exercise to strengthen joints.
Background
Osteoarthritis (OA) involves deterioration of the cartilage and interdependent joint tissues, including the bone.(2) Attributed to repeated stress on joint tissues, OA can occur in any joint but is particularly common in joints of the limbs, such as the knee, hip, hands (Fig. 1).(3) The risk factors for knee OA, reinforcing the role of stress, include exceptional bending such as required in some occupations, obesity, and knee surgery or other trauma.(4) The prevalence of OA climbs with age but is not confined to the elderly. According to U.S. data, the overall prevalence of OA in adults is 13.9% but reaches 33.6% in those aged 65 years or older. (5) The lifetime risk of OA, which is a leading cause of disability,(6) is 44.7%, rising to 56.8% in those with a history of knee injury.(7)
In Canada, arthritis of all types collectively represents the third most common chronic condition with a peak prevalence in late middle age (Fig. 2A) and (Fig. 2B). (8) Of the arthritis types and joints affected, knee OA represents a large and increasing public health burden due to rising rates of obesity and an aging population.(9) The estimated costs are substantial, stemming not only from healthcare services for OA, which is the most frequent indication for knee replacement,(10) but restrictions imposed by the disease on normal function, including occupational activities.(11,12) Evidence that symptomatic knee OA has been increasing at rates not fully explained by rising rates of obesity emphasizes the need for structured use of rational management strategies.(13)
Knee OA Pathology and Risk of Progression
In patients with knee OA, structural joint deterioration is the common characteristic within a broad range of clinical and radiological presentations. Focal areas of abnormality may affect a range of tissues other than hyaline articular cartilage, including ligaments and bone.(14) When cartilage loss is sufficient, bony remodeling may occur, producing malalignment.(15) This malalignment can trigger further cartilage loss and damage that underlies progression (Fig. 3). The risk and speed of progression is variable. In some individuals, symptoms remain relatively stable for extended periods. In others, synovitis and other forms of inflammation exacerbate joint deterioration, leading eventually to disability best relieved with joint replacement.(16)
Radiological findings vary markedly and may not uniformly correlate with symptoms.(17) Several radiographic grading systems for assessing the severity of knee OA have been proposed. Of these, the Kellgren and Lawrence classifications, which were described more than 40 years ago,(18) are typical and remain widely used. In this system, the four classifications after grade 0 (no abnormality) range from modest osteophytic lipping (grade 1) to deformity of the bone contour (grade 4) (Table 1).
The cardinal signs of knee OA, according to guidelines from the American College of Radiology (ACR) and others, include pain, transient morning stiffness, and crepitus on motion.(19) The accuracy of a physical examination for making the diagnosis of knee OA increases with other classic features, such as bony tenderness and malalignment,(20) but imaging will be useful in the substantial minority of patients with an atypical presentation.(21) Although radiographs may be normal in patients with early OA,(22) other types of imaging studies, such as MRI and ultrasound, can provide additional information when used alone or in combination.(23) Laboratory tests are not useful for the diagnosis of OA but they may be of value for ruling out alternative diagnoses, such as gout or infection.
Managing OA: Goals of Therapy
The goal of treatment is to alleviate symptoms and prevent progression. A combination of pharmacological and non-pharmacological treatments is typically appropriate. There is no cure for knee OA, but treatments to improve quality of life and slow or halt progression of joint deterioration can be expected to yield both immediate and long-term benefits.
Among oral therapies for symptom control, nonsteroidal anti-inflammatory drugs (NSAIDs) are modestly more effective than acetaminophen,(24) but the toxicity of NSAIDs, including COX- 2 inhibitors, is cumulative.(25,26) Strategies to reduce the gastrointestinal effects of non-selective NSAIDs by agents that suppress gastric acid have been effective,(27) but long-term use of both COX- 2 inhibitors and NSAIDs pose risks, particularly nephrotoxicity.(28,29) Glucosamine and chondroitin are safer but appear to be even less effective than simple analgesics. Although several relatively small controlled trials have associated these agents with benefit,(30,31) a multicenter trial with four arms was unable to show a statistical advantage for either glucosamine or chondroitin relative to placebo after 24 weeks of therapy.(32)
Of intra-articular injections, there is evidence of benefit from both corticosteroids and from hyaluronic acid (HA). For corticosteroids, pain relief is relatively rapid and attributed to the anti-inflammatory effect, but there appears to be diminishing benefit over time.(33) In a meta-analysis the long-term safety of corticosteroids were found acceptable,(34) but prolonged use does raise theoretical concerns from such issues as change in immune function.(35) Several guidelines, including those from the American Academy of Orthopedic Surgeons (AAOS), do not currently recommend intra-articular corticosteroids for routine care of knee OA.(36)
Intra-articular injection of HA, which is an endogenous glycosaminoglycan found in several tissues of the body, received regulatory approval for the treatment of knee OA almost 20 years ago and is widely used due to a favorable benefit-to-risk ratio. In the knee, endogenous HA is associated with providing viscoelasticity to the synovial fluid, improving distribution of stress.(37) Experimental studies suggest HA may play an active role in chondrocyte repair and knee stability.(38) Clinical studies support longer-term benefit from HA relative to intraarticular corticosteroids and pain relief comparable to NSAIDs.(39) While some patients experience pain on injection, the safety profile of HA is otherwise comparable to placebo.(40)
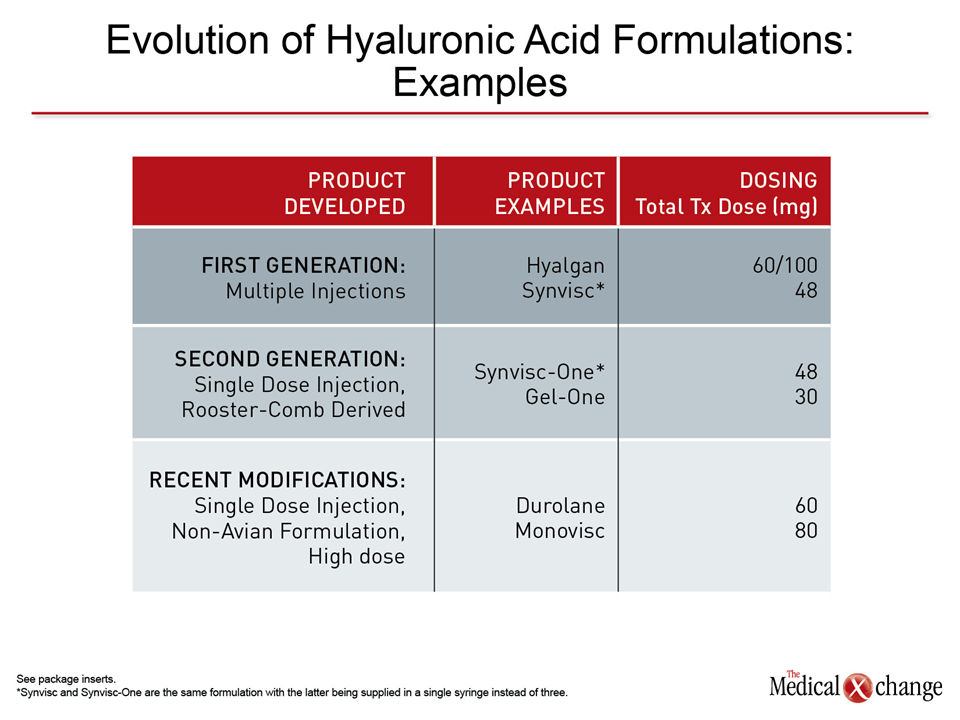
HA preparations may not be interchangeable. Since the first commercial preparations were made available, subsequent products have been developed for improved safety, efficacy, or both. For example, all initial products were formulated from rooster combs. The avian origin generated labeling that advised caution in individuals allergic to poultry or poultry products. While many newer products are non-avian based, they have the additional advantage of extended activity. The newest non-avian HA products, such as Monovisc and Durolane, are associated with efficacy for up to six months. While this is an advantage for patients with a low tolerance for injections, the longer intervals suggest greater potency of effect that may also be relevant to speed of onset. Relative to initial formulations, many of the recent HA preparations have high molecular weights, which may offer greater potency for tissue repair.(41) Lastly, HA dose formulations vary (Table 2).
Of other pharmacologic options for the treatment of knee OA, most, such as opiates, are used for short-term symptom control. While the theoretical value of HA in restoring normal physiology to halt progression is intriguing, there is no evidence that any drug therapy reverses knee OA progression.
For patients presenting with symptomatic OA, pharmacologic therapy is first-line therapy for symptom control, but non-pharmacological therapy may play an essential role in slowing or preventing disease progression. In patients with knee OA associated with obesity, for example, weight loss is strongly recommended in the AAOS guidelines.(36)
The risks of excess body fat not only include mechanical load but upregulation of proinflammatory factors that may contribute to joint pathology.(42) Obesity has also been associated with increased pain perception independent of joint weight bearing.(43) Loss of weight in obese individuals with knee OA has been associated with both symptom relief and slower disease progression.(44)
Strengthening muscles, particularly quadriceps, is also an effective non-pharmacologic approach to improved joint function.(45) In controlled trials, aerobic and resistance exercises as well as strength training have all been found effective for reducing symptoms of OA with the potential to slow progression.(46,47) As malalignment is considered an important predictor of progression, braces, orthotics, and compression sleeves all have potential benefit in selected patients. In a study of patients with varus malalignment, knee pain was reduced relative to no therapy with a neoprene sleeve.(48) In another study, also recruiting patients with varus malalignment, pain relief was achieved with a lateral wedge orthotic.(49)
Although potentially useful for specific indications, surgical treatments for knee OA have been largely disappointing. In a meta-analysis, joint lavage was not associated with either improved joint function or reduced pain.(50) A similar conclusion was drawn from a meta-analysis of studies of arthroscopic debridement.(51) In a review of other types of surgeries for knee OA, such as osteotomy or joint fusion, none were considered to be appropriate for routine use in European consensus guidelines.(52) Surgical procedures performed for specific goals, such as arthroscopic removal of loose bodies may be appropriate in selected patients, but the value of surgery other than total joint replacement has not been well documented.
A better understanding of the pathophysiology of knee OA is urgently needed to improve therapeutic options. While many of the etiologies of OA are well described, including mechanical joint stress and genetic susceptibility,(53) the specific molecular events that sustain cartilage deterioration remain poorly understood. Targeted therapies in OA as in other diseases may provide the greatest opportunity for tissue repair or regeneration. Autologous chondrocyte implantation is among several techniques with promise to produce durable cartilage repair.(54) Efforts to increase the biologic activity of exogenously administered HA, which has been shown to regulate chondrocyte behavior in experimental studies,(55) is another. Such treatments could play a critical role in lessening the growing burden of knee OA.
Conclusion
Knee OA increases in prevalence with age but is a common source of functional impairments and diminished quality of life even in relatively young and otherwise healthy patients. Early initiation of therapy has the potential to attenuate disease progression when pharmacologic and non-pharmacologic management is combined to control symptoms and modify factors likely to exacerbate joint deterioration. Due to the chronicity of knee OA, the most attractive pharmacologic options are those that pose the lowest cumulative risk of toxicity. NSAIDs and HA have both demonstrated efficacy in knee OA but offer unequal risks of adverse events. The potential for HA to improve joint function over time remains a theoretical reason to consider long-acting and high-dose formulations of agents in this drug class. The search for additional agents with an ability to attenuate the disease process is underway.
References
1. Life with Arthritis in Canada. Public Health Agency of Canada; 2010.
2. DiCesare P, Abramson S, Samuels J. Pathogenesis of Osteoarthritis. In: Firestein GS, Kelly WN, eds. Kelley’s Textbook of Rheumatology. Philadelphia: Saunders Elsevier; 2009:1425-540.
3. Wood AM, Brock TM, Heil K, Holmes R, Weusten A. A review on the management of hip and knee osteoarthritis. International Journal of Chronic Diseases 2013;2:1-10.
4. Felson DT. Epidemiology of Osteoarthritis. In: Brandt KD, Doherty M, Lohmander LS, eds. Osteoarthritis. Oxford, England: Oxford Press; 2003:9-16.
5. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and rheumatism 2008;58:26-35.
6. Centers for Disease C, Prevention. Prevalence and most common causes of disability among adults–United States, 2005. MMWR Morbidity and mortality weekly report 2009;58:421-6.
7. Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis and rheumatism 2008;59:1207-13.
8. PHAC. Life with Arthritis in Canada. http://wwwphac-aspcgcca/ cd-mc/arthritis-arthrite/lwaic-vaaac-10/3-engphp – f12: Public Health Agency of Canada; 2014.
9. ASC. Arthritis Facts and Statistics. Arthritis Society of Canada; 2014.
10. Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. The Journal of bone and joint surgery American volume 2013;95:385-92.
11. Badley EM, Wang PP. The contribution of arthritis and arthritis disability to nonparticipation in the labor force: a Canadian example. The Journal of rheumatology 2001;28:1077-82.
12. Gignac MA, Cao X, Lacaille D, Anis AH, Badley EM. Arthritisrelated work transitions: a prospective analysis of reported productivity losses, work changes, and leaving the labor force. Arthritis and rheumatism 2008;59:1805-13.
13. Nguyen US, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Annals of internal medicine 2011;155:725-32.
14. Felson DT. Clinical practice. Osteoarthritis of the knee. The New England journal of medicine 2006;354:841-8.
15. Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA : the journal of the American Medical Association 2001;286:188-95.
16. Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis and rheumatism 2001;44:1237-47.
17. D’Ambrosia RD. Epidemiology of osteoarthritis. Orthopedics 2005;28:s201-5.
18. Kellgren JH , Lawrence JS. Radiological assessment of osteoarthrosis. Annals of the rheumatic diseases 1957;16:494-502.
19. Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis and rheumatism 1986;29:1039-49.
20. Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Annals of the rheumatic diseases 2010;69:483-9.
21. Cibere J, Bellamy N, Thorne A, et al. Reliability of the knee examination in osteoarthritis: effect of standardization. Arthritis and rheumatism 2004;50:458-68.
22. Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. The Journal of rheumatology 2000;27:1513-7.
23. Braun HJ , Gold GE. Diagnosis of osteoarthritis: imaging. Bone 2012;51:278-88.
24. Pincus T, Koch GG, Sokka T, et al. A randomized, double-blind, crossover clinical trial of diclofenac plus misoprostol versus acetaminophen in patients with osteoarthritis of the hip or knee. Arthritis and rheumatism 2001;44:1587-98.
25. Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. The New England journal of medicine 1999;340:1888-99.
26. Fitzgerald GA. Coxibs and cardiovascular disease. The New England journal of medicine 2004;351:1709-11.
27. Yeomans ND, Tulassay Z, Juhasz L, et al. A comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiinflammatory drugs. Acid Suppression Trial: Ranitidine versus Omeprazole for NSAID-associated Ulcer Treatment (ASTRONAUT) Study Group. The New England journal of medicine 1998;338:719-26.
28. Knights KM, Tsoutsikos P, Miners JO. Novel mechanisms of nonsteroidal anti-inflammatory drug-induced renal toxicity. Expert opinion on drug metabolism & toxicology 2005;1:399-408.
29. Harris RC, Breyer MD. Update on cyclooxygenase-2 inhibitors. Clinical journal of the American Society of Nephrology : CJASN 2006;1:236-45.
30. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. The Cochrane database of systematic reviews 2005:CD005321.
31. McAlindon TE, LaValley MP, Felson DT. Efficacy of glucosamine and chondroitin for treatment of osteoarthritis. JAMA : the journal of the American Medical Association 2000;284:1241.
32. Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. The New England journal of medicine 2006;354:795-808.
33. Hepper CT, Halvorson JJ , Duncan ST, Gregory AJ, Dunn WR, Spindler KP. The efficacy and duration of intra-articular corticosteroid injection for knee osteoarthritis: a systematic review of level I studies. The Journal of the American Academy of Orthopaedic Surgeons 2009;17:638-46.
34. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. The Cochrane database of systematic reviews 2005:CD005328.
35. Marsland D, Mumith A, Barlow IW. Systematic review: the safety of intra-articular corticosteroid injection prior to total knee arthroplasty. The Knee 2014;21:6-11.
36. AAOS. Treatment of Osteoarthritis of the Knee. http:// wwwaaosorg/Research/guidelines TreatmentofOsteoarthritis oftheKneeGuidelinepdf: American Academy of Orthopaedic Surgery (AAOS); 2013.
37. Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis research & therapy 2003;5:54-67.
38. Nehrer S, Domayer S, Dorotka R, Schatz K, Bindreiter U, Kotz R. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. European journal of radiology 2006;57:3-8.
39. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. The Cochrane database of systematic reviews 2006:CD005321.
40. Miller LE, Block JE. US-Approved Intra-Articular Hyaluronic Acid Injections are Safe and Effective in Patients with Knee Osteoarthritis: Systematic Review and Meta-Analysis of Randomized, Saline-Controlled Trials. Clinical medicine insights Arthritis and musculoskeletal disorders 2013;6:57-63.
41. Wang CT, Lin YT, Chiang BL, Lin YH , Hou SM. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2006;14:1237-47.
42. Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Current opinion in rheumatology 2010;22:533-7.
43. Heim N, Snijder MB, Deeg DJ, Seidell JC, Visser M. Obesity in older adults is associated with an increased prevalence and incidence of pain. Obesity 2008;16:2510-7.
44. Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis and rheumatism 2004;50:1501-10.
45. Hurley MV, Newham DJ. The influence of arthrogenous muscle inhibition on quadriceps rehabilitation of patients with early, unilateral osteoarthritic knees. British journal of rheumatology 1993;32:127-31.
46. Ettinger WH, Jr., Burns R, Messier SP, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST). JAMA : the journal of the American Medical Association 1997;277:25-31.
47. Baker KR, Nelson ME, Felson DT, Layne JE, Sarno R, Roubenoff R. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. The Journal of rheumatology 2001;28:1655-65.
48. Kirkley A, Webster-Bogaert S, Litchfield R, et al. The effect of bracing on varus gonarthrosis. The Journal of bone and joint surgery American volume 1999;81:539-48.
49. Maillefert JF , Hudry C, Baron G, et al. Laterally elevated wedged insoles in the treatment of medial knee osteoarthritis: a prospective randomized controlled study. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2001;9:738-45.
50. Reichenbach S, Rutjes AW, Nuesch E, Trelle S, Juni P. Joint lavage for osteoarthritis of the knee. The Cochrane database of systematic reviews 2010:CD007320.
51. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. The Cochrane database of systematic reviews 2008:CD005118.
52. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 2008;16:137-62.
53. Stewart TL, Ralston SH. Role of genetic factors in the pathogenesis of osteoporosis. The Journal of endocrinology 2000;166:235-45.
54. Hangody L, Vasarhelyi G, Hangody LR, et al. Autologous osteochondral grafting–technique and long-term results. Injury 2008;39 Suppl 1:S32-9.
55. Ishida O, Tanaka Y, Morimoto I, Takigawa M, Eto S. Chondrocytes are regulated by cellular adhesion through CD44 and hyaluronic acid pathway. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 1997;12:1657-63.
Chapter 1: Osteoarthritis of the Knee: Addressing a Growing Epidemic
Osteoarthritis (OA) of the knee is a common orthopedic complaint, affecting about one in six adult Canadians.(1) A chronic and progressive inflammatory condition, OA is painful, restricts activity, and impairs quality of life. Due to its chronicity, it is essential to consider the long-term safety and tolerability of therapeutic options in the context of extended symptom control. Strategies to slow the pace of joint deterioration are being pursued, but the current focus of intervention is on symptom control. Traditionally, such simple analgesics as acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) have been the first-line option, but their efficacy is limited and there are safety concerns with chronic use. For localized joint injections, hyaluronic acid (HA), which is a component of normal joint physiology, has safety advantages over corticosteroids, particularly when repeated treatments are anticipated. The durability of the effect of hyaluronic acid is also an advantage relative to other options. The success of any therapy is likely to be increased with non-pharmacologic support, particularly exercise to strengthen joints.
Show review